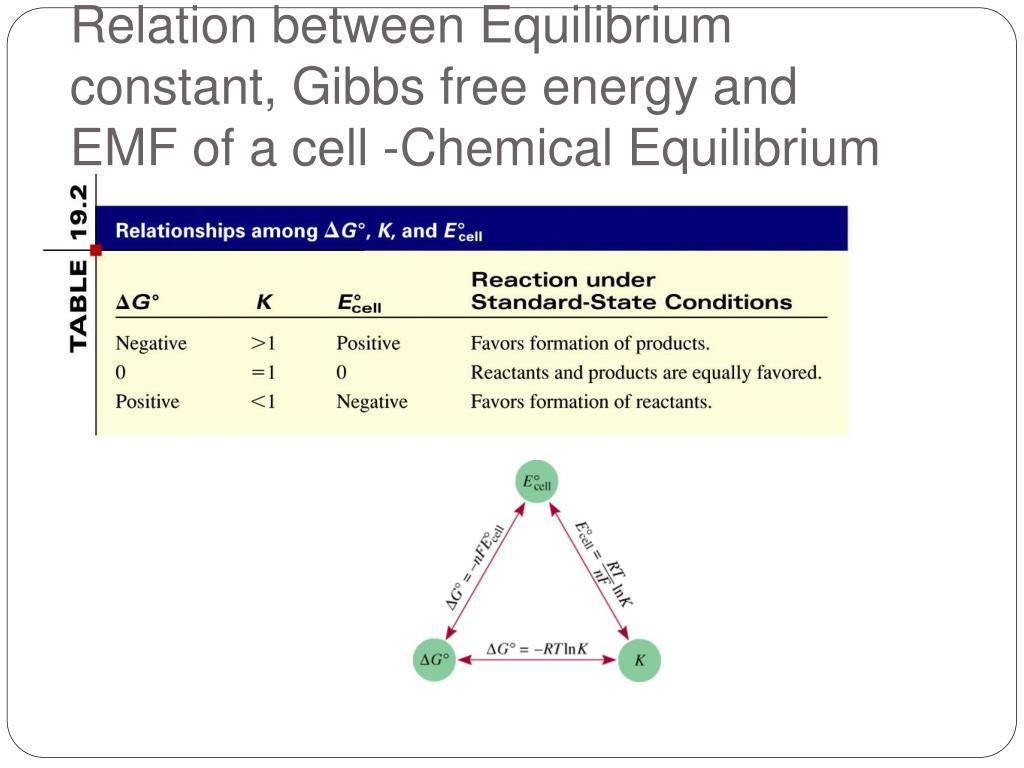
Electrochemical Cells Equilibrium Constant . Electrochemical cells convert chemical energy to electrical energy and vice versa. Electrochemical cells as chemical probes 2. The standard state potential for a cell. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The nernst equation is used in. The actual relationship between the free energy change and the cell potential is given by the following formula: The total amount of energy produced by. Where n is the number of moles of electrons. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical.
from www.slideserve.com
The total amount of energy produced by. The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Electrochemical cells convert chemical energy to electrical energy and vice versa. Where n is the number of moles of electrons. To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. Electrochemical cells as chemical probes 2.
PPT Electrochemistry MAE295 PowerPoint Presentation, free download
Electrochemical Cells Equilibrium Constant The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The actual relationship between the free energy change and the cell potential is given by the following formula: To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. Electrochemical cells as chemical probes 2. The standard state potential for a cell. Electrochemical cells convert chemical energy to electrical energy and vice versa. Where n is the number of moles of electrons. The total amount of energy produced by. The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Electrochemical Cells Equilibrium Constant The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The standard state potential for a cell. To calculate the equilibrium constant for. Electrochemical Cells Equilibrium Constant.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrochemical Cells Equilibrium Constant The actual relationship between the free energy change and the cell potential is given by the following formula: The standard state potential for a cell. The total amount of energy produced by. To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa +. Electrochemical Cells Equilibrium Constant.
From app.jove.com
Gibbs Free Energy, Cell Potential and Equilibrium Constant Concept Electrochemical Cells Equilibrium Constant In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Electrochemical cells as chemical probes 2. The actual relationship between the free energy change and the cell potential is given by the following formula: Electrochemical cells convert chemical energy to electrical energy and vice versa. The. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Class 12 Electrochemistry ( How to calculate equilibrium constant by Electrochemical Cells Equilibrium Constant The actual relationship between the free energy change and the cell potential is given by the following formula: Electrochemical cells as chemical probes 2. Where n is the number of moles of electrons. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. In this section we. Electrochemical Cells Equilibrium Constant.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Electrochemical Cells Equilibrium Constant The total amount of energy produced by. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. Electrochemical cells convert chemical energy to electrical energy and vice versa. To calculate the equilibrium constant for an electrochemical cell we need to know: In this section we will introduce. Electrochemical Cells Equilibrium Constant.
From saylordotorg.github.io
Describing Electrochemical Cells Electrochemical Cells Equilibrium Constant The standard state potential for a cell. Where n is the number of moles of electrons. Electrochemical cells as chemical probes 2. The actual relationship between the free energy change and the cell potential is given by the following formula: The total amount of energy produced by. To calculate the equilibrium constant for an electrochemical cell we need to know:. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED A student made measurements on some electrochemical cells and Electrochemical Cells Equilibrium Constant Electrochemical cells as chemical probes 2. The standard state potential for a cell. To calculate the equilibrium constant for an electrochemical cell we need to know: The actual relationship between the free energy change and the cell potential is given by the following formula: Where n is the number of moles of electrons. In this section we will introduce the. Electrochemical Cells Equilibrium Constant.
From saylordotorg.github.io
Electrochemistry Electrochemical Cells Equilibrium Constant The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. To calculate the equilibrium constant for an electrochemical cell we need to know: The. Electrochemical Cells Equilibrium Constant.
From www.slideserve.com
PPT Electrochemistry MAE295 PowerPoint Presentation, free download Electrochemical Cells Equilibrium Constant The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Electrochemical cells as chemical probes 2. The standard state potential for a cell.. Electrochemical Cells Equilibrium Constant.
From scoop.eduncle.com
62. the value of the equilibrium constant of an electrochemical cell Electrochemical Cells Equilibrium Constant The actual relationship between the free energy change and the cell potential is given by the following formula: In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The total amount of energy produced by. The nernst equation is used in. To calculate the equilibrium constant. Electrochemical Cells Equilibrium Constant.
From www.chegg.com
Solved What is the equilibrium constant K at 25°C for an Electrochemical Cells Equilibrium Constant To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The total amount of energy produced by. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Electrochemical cells convert chemical energy to electrical energy and. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED student made measurements on some ectrochemica cells and Electrochemical Cells Equilibrium Constant In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Electrochemical cells as chemical probes 2. The actual relationship between the free energy change and the cell potential is given by the following formula: The equilibrium constant \(k\) is the equilibrium constant of a general reaction. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Electrochemistry and Free Energy, Nernst Equation, Equilibrium Constant Electrochemical Cells Equilibrium Constant Electrochemical cells convert chemical energy to electrical energy and vice versa. Electrochemical cells as chemical probes 2. Where n is the number of moles of electrons. The actual relationship between the free energy change and the cell potential is given by the following formula: The total amount of energy produced by. The standard state potential for a cell. The equilibrium. Electrochemical Cells Equilibrium Constant.
From slideplayer.com
Electrochemistry. ppt download Electrochemical Cells Equilibrium Constant The total amount of energy produced by. Electrochemical cells convert chemical energy to electrical energy and vice versa. The nernst equation is used in. The standard state potential for a cell. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. Where n is the number of. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Electrochemistry 10 Relationship between Nernst Equation and Electrochemical Cells Equilibrium Constant The actual relationship between the free energy change and the cell potential is given by the following formula: In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Electrochemical Cells Equilibrium Constant To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. Where n is the number of moles of electrons. The nernst equation is used in. Electrochemical cells as chemical probes 2. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc +. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Nernst equation applications How to calculate equilibrium constant Electrochemical Cells Equilibrium Constant In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. To calculate the equilibrium constant for an electrochemical cell we need to know: The actual relationship between the free energy change and the cell potential is given by the following formula: The standard state potential for. Electrochemical Cells Equilibrium Constant.
From app.jove.com
Gibbs Free Energy, Cell Potential and Equilibrium Constant Chemistry Electrochemical Cells Equilibrium Constant The standard state potential for a cell. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb. Electrochemical Cells Equilibrium Constant.
From www.slideserve.com
PPT Chapter 10 Equilibrium Electrochemistry PowerPoint Presentation Electrochemical Cells Equilibrium Constant The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. To calculate the equilibrium constant for an electrochemical cell we need to know: Electrochemical. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for Electrochemical Cells Equilibrium Constant The standard state potential for a cell. The actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation is used in. To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED made measurements on some electrochemical cells and calculated Electrochemical Cells Equilibrium Constant In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The standard state potential for a cell. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. The actual relationship between the free. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED A student made measurements on some electrochemical cells and Electrochemical Cells Equilibrium Constant Where n is the number of moles of electrons. The standard state potential for a cell. The nernst equation is used in. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. Electrochemical cells as chemical probes 2. In this section we will introduce the electrochemical potential. Electrochemical Cells Equilibrium Constant.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID Electrochemical Cells Equilibrium Constant Electrochemical cells convert chemical energy to electrical energy and vice versa. The actual relationship between the free energy change and the cell potential is given by the following formula: Where n is the number of moles of electrons. The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED What is the equilibrium constant K at 25°C for an Electrochemical Cells Equilibrium Constant The nernst equation is used in. To calculate the equilibrium constant for an electrochemical cell we need to know: Electrochemical cells convert chemical energy to electrical energy and vice versa. The standard state potential for a cell. Where n is the number of moles of electrons. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
What is the equilibrium constant K at 25°C for an electrochemical cell Electrochemical Cells Equilibrium Constant The total amount of energy produced by. Where n is the number of moles of electrons. The nernst equation is used in. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. Electrochemical cells as chemical probes 2. The equilibrium constant of an electrochemical cell's redox reaction. Electrochemical Cells Equilibrium Constant.
From www.toppr.com
Given the equilibrium constant, Kc of the reaction Cu(s) + 2Ag^ + (aq Electrochemical Cells Equilibrium Constant Where n is the number of moles of electrons. The total amount of energy produced by. The standard state potential for a cell. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. Electrochemical cells as chemical probes 2. In this section we will introduce the electrochemical. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Electrochemistry 5 Nernst Equation Equilibrium Constant Gibb’s Electrochemical Cells Equilibrium Constant Electrochemical cells as chemical probes 2. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. Where n is the number of moles of electrons. To calculate the equilibrium constant for an electrochemical cell we need to know: The nernst equation is used in. In this section. Electrochemical Cells Equilibrium Constant.
From www.slideserve.com
PPT Electrochemistry MAE 212 PowerPoint Presentation, free download Electrochemical Cells Equilibrium Constant To calculate the equilibrium constant for an electrochemical cell we need to know: The total amount of energy produced by. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The actual relationship between the free energy change and the cell potential is given by the following. Electrochemical Cells Equilibrium Constant.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Electrochemical Cells Equilibrium Constant The nernst equation is used in. The equilibrium constant of an electrochemical cell's redox reaction can be calculated using the nernst equation and the relationship between standard cell potential. The standard state potential for a cell. To calculate the equilibrium constant for an electrochemical cell we need to know: The equilibrium constant \(k\) is the equilibrium constant of a general. Electrochemical Cells Equilibrium Constant.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material Electrochemical Cells Equilibrium Constant The actual relationship between the free energy change and the cell potential is given by the following formula: In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The standard state potential for a cell. The nernst equation is used in. The equilibrium constant \(k\) is. Electrochemical Cells Equilibrium Constant.
From www.slideserve.com
PPT Chapter 8 Electrochemical Cells PowerPoint Presentation, free Electrochemical Cells Equilibrium Constant Where n is the number of moles of electrons. The actual relationship between the free energy change and the cell potential is given by the following formula: The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The total amount of energy produced by. To calculate the. Electrochemical Cells Equilibrium Constant.
From www.numerade.com
SOLVED student made measurements on some electrochemical cells and Electrochemical Cells Equilibrium Constant The standard state potential for a cell. Electrochemical cells convert chemical energy to electrical energy and vice versa. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb \leftrightharpoons cc + dd \label{6} \] and. The total amount of energy produced by. Where n is the number of moles of electrons. To calculate the. Electrochemical Cells Equilibrium Constant.
From slideplayer.com
Electrochemical Cell. ppt download Electrochemical Cells Equilibrium Constant Electrochemical cells as chemical probes 2. The standard state potential for a cell. To calculate the equilibrium constant for an electrochemical cell we need to know: The total amount of energy produced by. Electrochemical cells convert chemical energy to electrical energy and vice versa. The equilibrium constant \(k\) is the equilibrium constant of a general reaction \[ aa + bb. Electrochemical Cells Equilibrium Constant.
From www.youtube.com
Week 13 10. Calculating an equilibrium constant, K, from cell Electrochemical Cells Equilibrium Constant The standard state potential for a cell. Electrochemical cells as chemical probes 2. The actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of. Electrochemical Cells Equilibrium Constant.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium Electrochemical Cells Equilibrium Constant The total amount of energy produced by. Electrochemical cells convert chemical energy to electrical energy and vice versa. Where n is the number of moles of electrons. The actual relationship between the free energy change and the cell potential is given by the following formula: The standard state potential for a cell. To calculate the equilibrium constant for an electrochemical. Electrochemical Cells Equilibrium Constant.