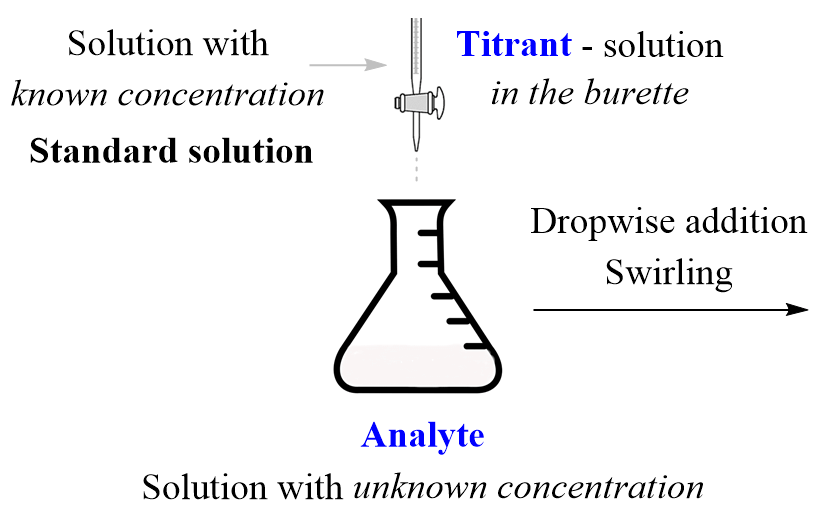
Titration Acid In Burette . Before the titration begins, the solution with the known concentration, also known. You have to decide if this experiment is suitable to use with different classes, and look at the need for. An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with an unknown molarity. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
from general.chemistrysteps.com
They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. An indicator is a dye added to a solution to change its color. Before the titration begins, the solution with the known concentration, also known. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix.
AcidBase Titrations Chemistry Steps
Titration Acid In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. Before the titration begins, the solution with the known concentration, also known. An indicator is a dye added to a solution to change its color. The analyte (titrand) is the solution with an unknown molarity. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
From scientificservices.eu
Titration Workstation UseScience Titration Acid In Burette Before the titration begins, the solution with the known concentration, also known. The analyte (titrand) is the solution with an unknown molarity. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. An indicator. Titration Acid In Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Acid In Burette An indicator is a dye added to a solution to change its color. You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The reagent (titrant) is the solution with a known molarity that will. Titration Acid In Burette.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titration Acid In Burette You have to decide if this experiment is suitable to use with different classes, and look at the need for. The analyte (titrand) is the solution with an unknown molarity. Before the titration begins, the solution with the known concentration, also known. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret. Titration Acid In Burette.
From www.istockphoto.com
Redox Titration With Burette And Pipette Stock Illustration Download Titration Acid In Burette The analyte (titrand) is the solution with an unknown molarity. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. An indicator is a dye added to a solution to change its color. slowly add the acid from the burette to the alkali in the conical flask, swirling. Titration Acid In Burette.
From stock.adobe.com
setup of titration acidbase called standardisation consisted of Titration Acid In Burette The analyte (titrand) is the solution with an unknown molarity. You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. An indicator is a dye added to a solution to. Titration Acid In Burette.
From medical-supplies-china.com
Acid burette PTFE piston glass burette laboratory titrationChina Titration Acid In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. You have to decide. Titration Acid In Burette.
From zamcopter.web.fc2.com
ACIDBASE TITRATION Titration Acid In Burette Before the titration begins, the solution with the known concentration, also known. An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Titration Acid In Burette.
From www.vrogue.co
Titration Apparatus vrogue.co Titration Acid In Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an unknown solution. Titration Acid In Burette.
From www.dreamstime.com
Redox Titration with Burette and Pipette Stock Vector Illustration of Titration Acid In Burette slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. in this experiment students. Titration Acid In Burette.
From vdocuments.mx
Acidbase titrations OH. Titration introduction Purpose Titration Acid In Burette Before the titration begins, the solution with the known concentration, also known. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the. Titration Acid In Burette.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titration Acid In Burette You have to decide if this experiment is suitable to use with different classes, and look at the need for. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. Before the titration begins, the solution with the known concentration, also known. The reagent (titrant) is the solution with a known molarity. Titration Acid In Burette.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. You have to decide if this experiment is suitable to use. Titration Acid In Burette.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Acid In Burette The analyte (titrand) is the solution with an unknown molarity. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent (titrant) is the solution with a known. Titration Acid In Burette.
From www.lazada.com.ph
Acid burette transparent blue and white line PTFE piston acidbase dual Titration Acid In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Titration Acid In Burette.
From dxoenpeuk.blob.core.windows.net
Buret For Titration at Leonard Oconnell blog Titration Acid In Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. slowly add the acid from the burette. Titration Acid In Burette.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. Before the titration begins, the solution with the known concentration, also known. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a buret is primarily used for titration to determine the concentration. Titration Acid In Burette.
From avopix.com
AcidBase titration, neutralization reaction. Royalty Free Stock Titration Acid In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The analyte (titrand) is the solution with an unknown molarity. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. An indicator is a dye added to a solution to change. Titration Acid In Burette.
From mungfali.com
Acid Base Titration Calculation Titration Acid In Burette An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. in this experiment students neutralise sodium hydroxide with hydrochloric. Titration Acid In Burette.
From www.alamy.com
Titration experiment. Student taking a volume reading from a burette Titration Acid In Burette in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will. Titration Acid In Burette.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. Before the titration begins, the. Titration Acid In Burette.
From stock.adobe.com
Tripod, Laboratory Metal Burette Stand. Setup of titration acidbase Titration Acid In Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. Before the titration begins, the solution with the known concentration, also known. You have to decide if this experiment is suitable to use with. Titration Acid In Burette.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Acid In Burette The analyte (titrand) is the solution with an unknown molarity. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Acid In Burette.
From acidsandbasesfordummieschem.weebly.com
Titration Acid and Bases for Dummies! Titration Acid In Burette An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Acid In Burette.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Acid In Burette slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use. Titration Acid In Burette.
From www.alamy.com
Netherlands, highschool boy and girl using burette and Erlenmeyer flask Titration Acid In Burette Before the titration begins, the solution with the known concentration, also known. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with an unknown molarity. You have to decide if this experiment. Titration Acid In Burette.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Titration Acid In Burette You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Before the titration begins, the solution with the known concentration, also known. An indicator is a dye added to a. Titration Acid In Burette.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog Titration Acid In Burette You have to decide if this experiment is suitable to use with different classes, and look at the need for. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Before the titration begins, the solution with the known concentration, also known. slowly add the acid from the burette to the alkali in. Titration Acid In Burette.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Acid In Burette in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Before the titration begins, the solution with the known. Titration Acid In Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Acid In Burette You have to decide if this experiment is suitable to use with different classes, and look at the need for. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. slowly add. Titration Acid In Burette.
From iu.pressbooks.pub
Analysis of Acids and Bases by Titration IU East Experimental Titration Acid In Burette An indicator is a dye added to a solution to change its color. Before the titration begins, the solution with the known concentration, also known. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. You have to decide if this experiment is suitable to use with different classes, and. Titration Acid In Burette.
From exoxjybnj.blob.core.windows.net
Acid Base Titration Lab Introduction at Nichole Brumback blog Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. An indicator is a dye added to a solution to change its color. You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to. Titration Acid In Burette.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The analyte (titrand) is the solution with an unknown. Titration Acid In Burette.
From saylordotorg.github.io
AcidBase Titrations Titration Acid In Burette The reagent (titrant) is the solution with a known molarity that will react with the analyte. An indicator is a dye added to a solution to change its color. Before the titration begins, the solution with the known concentration, also known. The analyte (titrand) is the solution with an unknown molarity. They then concentrate the solution and allow it to. Titration Acid In Burette.
From www.amazon.com
Titration Equipment Set Complete Single Buret Burete Assembly with 100 Titration Acid In Burette slowly add the acid from the burette to the alkali in the conical flask, swirling to mix. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The reagent. Titration Acid In Burette.
From www.shutterstock.com
Lab Setup Burette Acidbase Titration Stock Vector 388181593 Shutterstock Titration Acid In Burette An indicator is a dye added to a solution to change its color. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Acid In Burette.