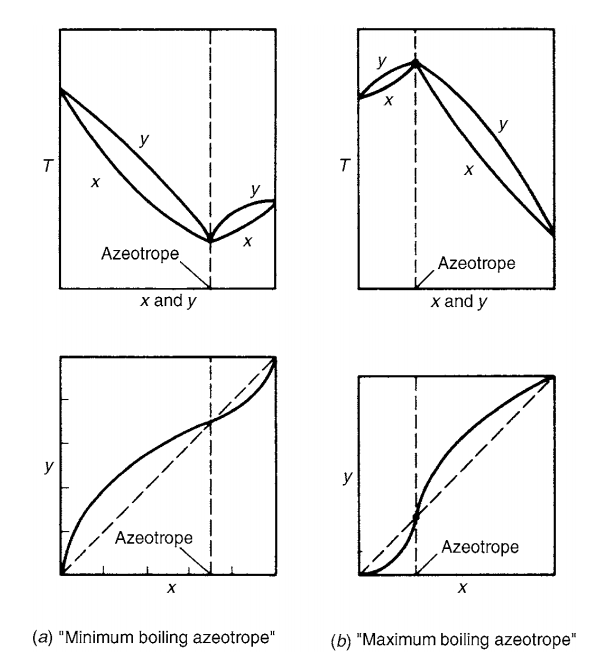
What Is The Boiling Point Of Ethanol Water Azeotrope . in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. Recognize and detect the effects of electrostatic charges on your balance. Azeotropes exist in solution at a boiling point specific for that component. the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The boiling point of this mixture is 78.2°c,. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. The next diagram shows the boiling point / composition curve for ethanol / water. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures.
from www.chemengghelp.com
The next diagram shows the boiling point / composition curve for ethanol / water. the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The boiling point of this mixture is 78.2°c,. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. Azeotropes exist in solution at a boiling point specific for that component. Recognize and detect the effects of electrostatic charges on your balance. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c.
Azeotropic Distillation Process for Ethanol Dehydration ChemEnggHelp
What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. Recognize and detect the effects of electrostatic charges on your balance. The boiling point of this mixture is 78.2°c,. The next diagram shows the boiling point / composition curve for ethanol / water. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. Azeotropes exist in solution at a boiling point specific for that component. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent.
From www.coursehero.com
[Solved] What is the composition of the azeotrope for the ethanol(1 What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. The next diagram shows the boiling point / composition curve for ethanol / water. The boiling point of this mixture is 78.2°c,. Azeotropes exist in solution at a boiling point specific for that component. in the case of mixtures of ethanol and water, this minimum occurs with 95.6%. What Is The Boiling Point Of Ethanol Water Azeotrope.
From school.careers360.com
azeotrope Overview, Structure, Properties & Uses What Is The Boiling Point Of Ethanol Water Azeotrope 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. Azeotropes exist in solution at a boiling point specific for that component. in fact, the most concentrated form of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.youtube.com
Miniumum Boiling Point Azeotropes Distillation (Lec 056) YouTube What Is The Boiling Point Of Ethanol Water Azeotrope in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. Recognize and detect the effects of electrostatic charges on your balance. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.slideserve.com
PPT Minimum Boiling Point Azeotrope PowerPoint Presentation, free What Is The Boiling Point Of Ethanol Water Azeotrope Azeotropes exist in solution at a boiling point specific for that component. The next diagram shows the boiling point / composition curve for ethanol / water. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. the boiling point of an azeotrope is either less than the boiling points of any. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.researchgate.net
Distillation of ethanolwater mixture. The most common processes are What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. Azeotropes exist in solution at a boiling point specific for that component. The boiling point of this mixture is 78.2°c,. Recognize and detect the effects of electrostatic charges on your balance. The next diagram shows the boiling. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.researchgate.net
Boiling points and molar fractions of the various azeotropes in the What Is The Boiling Point Of Ethanol Water Azeotrope Azeotropes exist in solution at a boiling point specific for that component. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. The boiling point of this mixture is 78.2°c,. You might think that this 0.3°c doesn't matter much, but it has huge. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.chembook.co.uk
chembook.co.uk CHEMISTRY IN PERSPECTIVE FOR BORED AND CONFUSED SENIOR What Is The Boiling Point Of Ethanol Water Azeotrope Azeotropes exist in solution at a boiling point specific for that component. The next diagram shows the boiling point / composition curve for ethanol / water. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The boiling point of this mixture is 78.2°c,. in fact, the most concentrated form of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.slideserve.com
PPT Minimum Boiling Point Azeotrope PowerPoint Presentation, free What Is The Boiling Point Of Ethanol Water Azeotrope Azeotropes exist in solution at a boiling point specific for that component. Recognize and detect the effects of electrostatic charges on your balance. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. the boiling point of this mixture is 78.2°c, compared. What Is The Boiling Point Of Ethanol Water Azeotrope.
From jsmithmoore.com
Boiling point of ethanol celsius What Is The Boiling Point Of Ethanol Water Azeotrope The boiling point of this mixture is 78.2°c,. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. the. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.slideserve.com
PPT Minimum Boiling Point Azeotrope PowerPoint Presentation, free What Is The Boiling Point Of Ethanol Water Azeotrope The boiling point of this mixture is 78.2°c,. Recognize and detect the effects of electrostatic charges on your balance. The next diagram shows the boiling point / composition curve for ethanol / water. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. for example, a zeotropic. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.researchgate.net
Vapourliquid equilibrium of ethanolwater showing distillation steps What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. The boiling point of this mixture is 78.2°c,. The next diagram shows the boiling point / composition curve for ethanol / water. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From jsmithmoore.com
Boiling point of ethanol celsius What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The next diagram shows the boiling point / composition curve for ethanol / water. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. . What Is The Boiling Point Of Ethanol Water Azeotrope.
From school.careers360.com
azeotrope Overview, Structure, Properties & Uses What Is The Boiling Point Of Ethanol Water Azeotrope for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. the boiling point of an azeotrope is either less than the boiling points of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is The Boiling Point Of Ethanol Water Azeotrope The next diagram shows the boiling point / composition curve for ethanol / water. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. Azeotropes exist in solution at a boiling point specific for that component. the boiling point of an azeotrope is either less than. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The next diagram shows the boiling point / composition curve for ethanol / water. in the case of mixtures of ethanol and water, this minimum. What Is The Boiling Point Of Ethanol Water Azeotrope.
From visualacuities.blogspot.com
Azeotrope Ethanol Water Azeotrope What Is The Boiling Point Of Ethanol Water Azeotrope The boiling point of this mixture is 78.2°c,. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. Recognize and detect the effects of electrostatic charges on your balance. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. Azeotropes. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.youtube.com
Boiling Point for C2H5OH (Ethanol or Ethyl Alcohol) YouTube What Is The Boiling Point Of Ethanol Water Azeotrope The next diagram shows the boiling point / composition curve for ethanol / water. Azeotropes exist in solution at a boiling point specific for that component. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. Recognize and detect the effects of electrostatic charges on your balance.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.numerade.com
SOLVED 3. (3 points) Benzene and ethanol form an azeotropic mixture What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The boiling point of this mixture is 78.2°c,. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. 57 rows this page contains. What Is The Boiling Point Of Ethanol Water Azeotrope.
From sciencenotes.org
What Is an Azeotrope? Definition and Examples What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The boiling point of this mixture is 78.2°c,. The next diagram shows the boiling point / composition curve for ethanol / water. in the case of mixtures of ethanol and water, this minimum occurs with 95.6%. What Is The Boiling Point Of Ethanol Water Azeotrope.
From chemicaltweak.com
Azeotropic Distillation Process In Detail Binary Separation Technique What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. the boiling point of this mixture is 78.2°c, compared with the boiling point of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From foodtechnotes.com
Azeotropes Food Tech Notes What Is The Boiling Point Of Ethanol Water Azeotrope The boiling point of this mixture is 78.2°c,. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. Recognize and detect the effects of electrostatic charges on your balance. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.slideserve.com
PPT Minimum Boiling Point Azeotrope PowerPoint Presentation, free What Is The Boiling Point Of Ethanol Water Azeotrope for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. in the case of mixtures of ethanol and water,. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.chemengghelp.com
Azeotropic Distillation Process for Ethanol Dehydration ChemEnggHelp What Is The Boiling Point Of Ethanol Water Azeotrope You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. Recognize and detect the effects of electrostatic charges on your balance. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. Azeotropes exist in solution at. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.slideserve.com
PPT Topic 17 Equilibrium PowerPoint Presentation, free download ID What Is The Boiling Point Of Ethanol Water Azeotrope for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The next diagram shows the boiling point / composition curve for ethanol / water. . What Is The Boiling Point Of Ethanol Water Azeotrope.
From jsmithmoore.com
Boiling point of ethanol celsius What Is The Boiling Point Of Ethanol Water Azeotrope the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. The boiling point of this mixture is 78.2°c,. Recognize and detect the effects of electrostatic charges on your balance. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.numerade.com
Ethanolwater mixture at p = [vapor phase diagram of the Please; see What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. Azeotropes exist in solution at a boiling point specific for that component. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. the boiling point of an azeotrope is either less. What Is The Boiling Point Of Ethanol Water Azeotrope.
From byjus.com
17.Define azeotrope and its two types(maximum boiling azeotrope and What Is The Boiling Point Of Ethanol Water Azeotrope 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. for example, a zeotropic mixture of ethanol and water (about 96% ethanol and 4% water) boils at 78.174 °c, while pure ethanol boils at 78.3 °c. Azeotropes exist in solution at a boiling point specific for that component. Recognize and detect. What Is The Boiling Point Of Ethanol Water Azeotrope.
From foodtechnotes.com
Azeotropes Food Tech Notes What Is The Boiling Point Of Ethanol Water Azeotrope You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. the boiling point of an azeotrope is either less than the boiling points of any of its constituents (a positive azeotrope), or. in the case of mixtures of ethanol and water, this minimum occurs with 95.6%. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.researchgate.net
The effect of pressure on composition and temperature of azeotrope (a What Is The Boiling Point Of Ethanol Water Azeotrope The boiling point of this mixture is 78.2°c,. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. Recognize and detect the effects of electrostatic charges on your balance.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From jsmithmoore.com
Boiling point of ethanol celsius What Is The Boiling Point Of Ethanol Water Azeotrope in fact, the most concentrated form of ethanol, an azeotrope, is around 95.6% ethanol by weight because pure ethanol is basically nonexistent. The boiling point of this mixture is 78.2°c,. Recognize and detect the effects of electrostatic charges on your balance. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From www.numerade.com
SOLVED The following diagram shows the boiling curve What Is The Boiling Point Of Ethanol Water Azeotrope 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The boiling point of this mixture is 78.2°c,. Azeotropes exist in solution at a boiling point specific for that component. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From slideplayer.com
Lecture Notes Week 1 ChE 1008 Spring Term (032). ppt download What Is The Boiling Point Of Ethanol Water Azeotrope You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. Azeotropes exist in solution at a boiling point specific for that component. 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. for example, a zeotropic mixture of ethanol. What Is The Boiling Point Of Ethanol Water Azeotrope.
From mavink.com
Melting And Boiling Point Chart What Is The Boiling Point Of Ethanol Water Azeotrope Azeotropes exist in solution at a boiling point specific for that component. Recognize and detect the effects of electrostatic charges on your balance. You might think that this 0.3°c doesn't matter much, but it has huge implications for the separation of ethanol / water mixtures. the boiling point of an azeotrope is either less than the boiling points of. What Is The Boiling Point Of Ethanol Water Azeotrope.
From guidemanualephemerist.z14.web.core.windows.net
Ethanolwater Txy Diagram What Is The Boiling Point Of Ethanol Water Azeotrope 57 rows this page contains tables of azeotrope data for various binary and ternary mixtures of solvents. Recognize and detect the effects of electrostatic charges on your balance. The boiling point of this mixture is 78.2°c,. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c.. What Is The Boiling Point Of Ethanol Water Azeotrope.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Of Ethanol Water Azeotrope Recognize and detect the effects of electrostatic charges on your balance. in the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. the boiling point of this mixture is 78.2°c, compared with the boiling point of pure ethanol at 78.5°c, and water at 100°c. for example, a. What Is The Boiling Point Of Ethanol Water Azeotrope.