What Is Q In Specific Heat Capacity . Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. define heat capacity and specific heat capacity and differentiate between the two terms. q is the amount of heat we supply to a substance. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. Q = mcδt, where q is the symbol for heat transfer,. Deduce which substance will have. the quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. M is the mass of the substance we’re. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise.
from www.slideserve.com
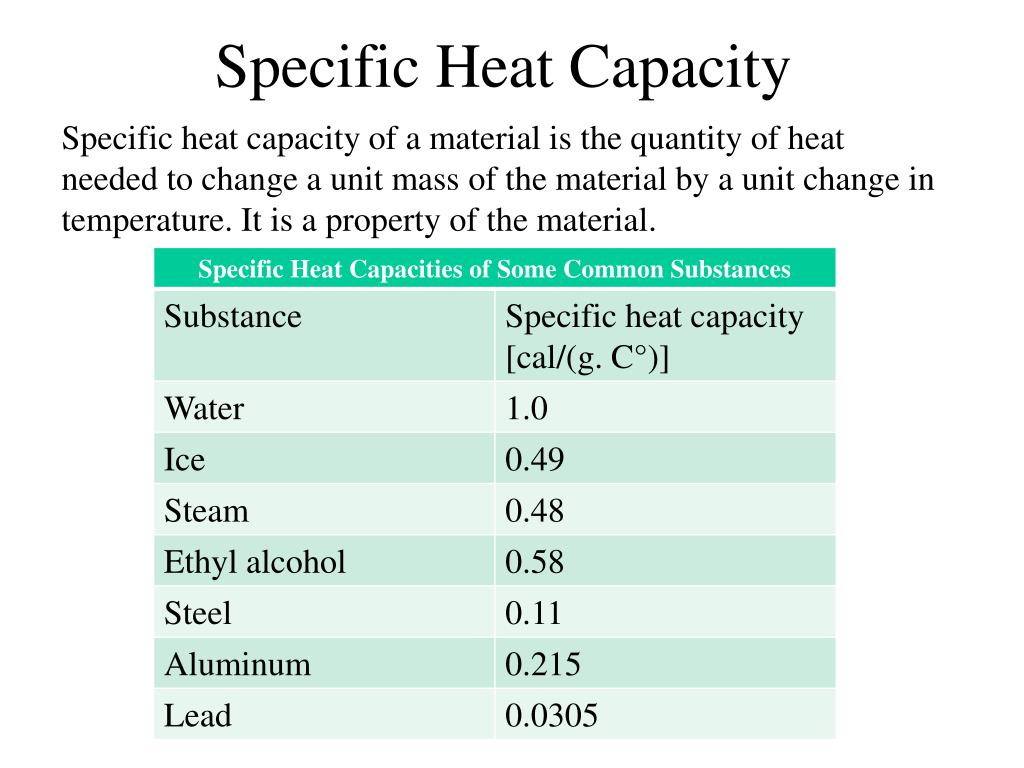
the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. q is the amount of heat we supply to a substance. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. Deduce which substance will have. The symbol c stands for. the quantitative relationship between heat transfer and temperature change contains all three factors: Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. define heat capacity and specific heat capacity and differentiate between the two terms. Q = mcδt, where q is the symbol for heat transfer,. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature.
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free
What Is Q In Specific Heat Capacity q is the amount of heat we supply to a substance. q is the amount of heat we supply to a substance. the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer,. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. M is the mass of the substance we’re. The symbol c stands for.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.3 Specific Heat Capacity翰林国际教育 What Is Q In Specific Heat Capacity q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. The symbol c stands for. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. Deduce which substance will have. M is the mass of the substance we’re. . What Is Q In Specific Heat Capacity.
From studylib.net
Heat Capacity of Metals PreLab What Is Q In Specific Heat Capacity The symbol c stands for. define heat capacity and specific heat capacity and differentiate between the two terms. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. q is the amount of heat we supply to a substance. Might be 1 j, 40 j,. What Is Q In Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Q In Specific Heat Capacity the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. The symbol c stands for. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. M is the mass of the substance we’re. the quantitative relationship between heat transfer and temperature. What Is Q In Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Q In Specific Heat Capacity Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. q is the amount of heat we supply to a substance. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. the quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c. What Is Q In Specific Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is Q In Specific Heat Capacity q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. define heat capacity and specific heat capacity and differentiate between the two terms. the specific heat. What Is Q In Specific Heat Capacity.
From studylib.net
Specific Heat Capacity (c) Formula q = mc)T Exercises (Be careful What Is Q In Specific Heat Capacity Deduce which substance will have. Q = mcδt, where q is the symbol for heat transfer,. q is the amount of heat we supply to a substance. the quantitative relationship between heat transfer and temperature change contains all three factors: the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity. What Is Q In Specific Heat Capacity.
From studylib.net
Heat Equation What Is Q In Specific Heat Capacity Q = mcδt, where q is the symbol for heat transfer,. The symbol c stands for. Deduce which substance will have. q is the amount of heat we supply to a substance. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Might be 1 j, 40 j, or even 50.000 j, any. What Is Q In Specific Heat Capacity.
From slidetodoc.com
SPECIFIC HEAT Calculations SPECIFIC HEAT CAPACITY The amount What Is Q In Specific Heat Capacity the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. q is the amount of heat we supply to a substance. define heat capacity and specific heat capacity and differentiate between the two terms. Q = mcδt, where q is the symbol for heat transfer,.. What Is Q In Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Q In Specific Heat Capacity define heat capacity and specific heat capacity and differentiate between the two terms. The symbol c stands for. Q = mcδt, where q is the symbol for heat transfer,. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Deduce which substance will have. the specific heat capacity (\ (c\)) of a. What Is Q In Specific Heat Capacity.
From byjus.com
The SI unit of specific heat capacity of a substance is What Is Q In Specific Heat Capacity the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. define heat capacity and specific heat capacity and differentiate between the two terms. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. Deduce which substance will. What Is Q In Specific Heat Capacity.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Q In Specific Heat Capacity q is the amount of heat we supply to a substance. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. the specific heat capacity (\ (c\)) of a substance,. What Is Q In Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity AQA GCSE Required Practical Follow Up YouTube What Is Q In Specific Heat Capacity Deduce which substance will have. q is the amount of heat we supply to a substance. M is the mass of the substance we’re. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. define heat capacity and specific heat capacity and differentiate between the two terms. the quantitative relationship between heat. What Is Q In Specific Heat Capacity.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! What Is Q In Specific Heat Capacity the quantitative relationship between heat transfer and temperature change contains all three factors: M is the mass of the substance we’re. Q = mcδt, where q is the symbol for heat transfer,. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. . What Is Q In Specific Heat Capacity.
From www.sliderbase.com
Specific Heat What Is Q In Specific Heat Capacity The symbol c stands for. M is the mass of the substance we’re. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. q is the amount of heat we supply to. What Is Q In Specific Heat Capacity.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Q In Specific Heat Capacity the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. M is the mass of the substance we’re. the specific heat capacity (\ (c\)) of a substance,. What Is Q In Specific Heat Capacity.
From testbook.com
Specific Heat Capacity Learning Notes for IIT JEE Testbook What Is Q In Specific Heat Capacity M is the mass of the substance we’re. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. the quantitative relationship between heat transfer. What Is Q In Specific Heat Capacity.
From mavink.com
Specific Heat Capacity Diagram What Is Q In Specific Heat Capacity The symbol c stands for. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. Q = mcδt, where q is the symbol for heat transfer,. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a. What Is Q In Specific Heat Capacity.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Is Q In Specific Heat Capacity the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. The symbol c stands for. M is the mass of the substance we’re. q is the amount of heat we supply to a substance. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. What Is Q In Specific Heat Capacity.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples What Is Q In Specific Heat Capacity the quantitative relationship between heat transfer and temperature change contains all three factors: q is the amount of heat we supply to a substance. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science,. What Is Q In Specific Heat Capacity.
From www.youtube.com
How to Calculate Specific Heat YouTube What Is Q In Specific Heat Capacity define heat capacity and specific heat capacity and differentiate between the two terms. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Deduce which substance will have. q. What Is Q In Specific Heat Capacity.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to What Is Q In Specific Heat Capacity M is the mass of the substance we’re. The symbol c stands for. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. the quantitative relationship between heat transfer and temperature change contains all three factors: Deduce which substance will have. the specific heat capacity is the heat or energy required to. What Is Q In Specific Heat Capacity.
From www.youtube.com
Specific heat capacity YouTube What Is Q In Specific Heat Capacity Q = mcδt, where q is the symbol for heat transfer,. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. M is the mass of the substance we’re.. What Is Q In Specific Heat Capacity.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Q In Specific Heat Capacity M is the mass of the substance we’re. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. The symbol c stands for. Q = mcδt, where q is the symbol for heat transfer,. Might be 1 j, 40 j, or even 50.000 j, any amount of joules. What Is Q In Specific Heat Capacity.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation What Is Q In Specific Heat Capacity the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise. Deduce which substance will have. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. q is the amount of heat we supply to a substance. Q = mcδt, where q. What Is Q In Specific Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Q In Specific Heat Capacity The symbol c stands for. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. q is the amount of heat we supply to a substance. Q = mcδt, where q is the symbol for heat transfer,. q = mcδt, where q is the symbol for heat transfer, m is the mass. What Is Q In Specific Heat Capacity.
From evelinrtzamora.blogspot.com
EvelinrtZamora What Is Q In Specific Heat Capacity Deduce which substance will have. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. Q = mcδt, where q is the symbol for heat transfer,. define heat capacity and specific heat capacity and differentiate between the two terms. q is the. What Is Q In Specific Heat Capacity.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Q In Specific Heat Capacity q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. The symbol c stands for. the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer,. the concept of. What Is Q In Specific Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Is Q In Specific Heat Capacity the quantitative relationship between heat transfer and temperature change contains all three factors: Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Q = mcδt, where q is the symbol for heat transfer,. the specific heat. What Is Q In Specific Heat Capacity.
From webapi.bu.edu
💌 Specific heat capacity of aluminum. Table of specific heat capacities What Is Q In Specific Heat Capacity q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. Deduce which substance will have. The symbol c stands for. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. the specific heat capacity is the heat or. What Is Q In Specific Heat Capacity.
From alianabilmccall.blogspot.com
Specific Heat Capacity Units AlianabilMccall What Is Q In Specific Heat Capacity Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. q is the amount of heat we supply to a substance. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the. What Is Q In Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Q In Specific Heat Capacity M is the mass of the substance we’re. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. Q = mcδt, where q is the symbol for heat transfer,. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. the quantitative. What Is Q In Specific Heat Capacity.
From studylib.net
1.3 Specific Heat Capacity What Is Q In Specific Heat Capacity the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. q is the amount of heat we supply to a substance. define heat capacity and specific heat capacity. What Is Q In Specific Heat Capacity.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA What Is Q In Specific Heat Capacity Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms. M is the mass of the substance we’re. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. The symbol c stands for. Q = mcδt, where q is the symbol for heat transfer,. q. What Is Q In Specific Heat Capacity.
From physicscalculations.com
How to Calculate Specific Heat Capacity What Is Q In Specific Heat Capacity M is the mass of the substance we’re. Might be 1 j, 40 j, or even 50.000 j, any amount of joules go. Q = mcδt, where q is the symbol for heat transfer,. the concept of specific heat capacity, a fundamental principle in thermodynamics and material science, it. q is the amount of heat we supply to. What Is Q In Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube What Is Q In Specific Heat Capacity Q = mcδt, where q is the symbol for heat transfer,. the specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant. q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and δt is the change in temperature. . What Is Q In Specific Heat Capacity.