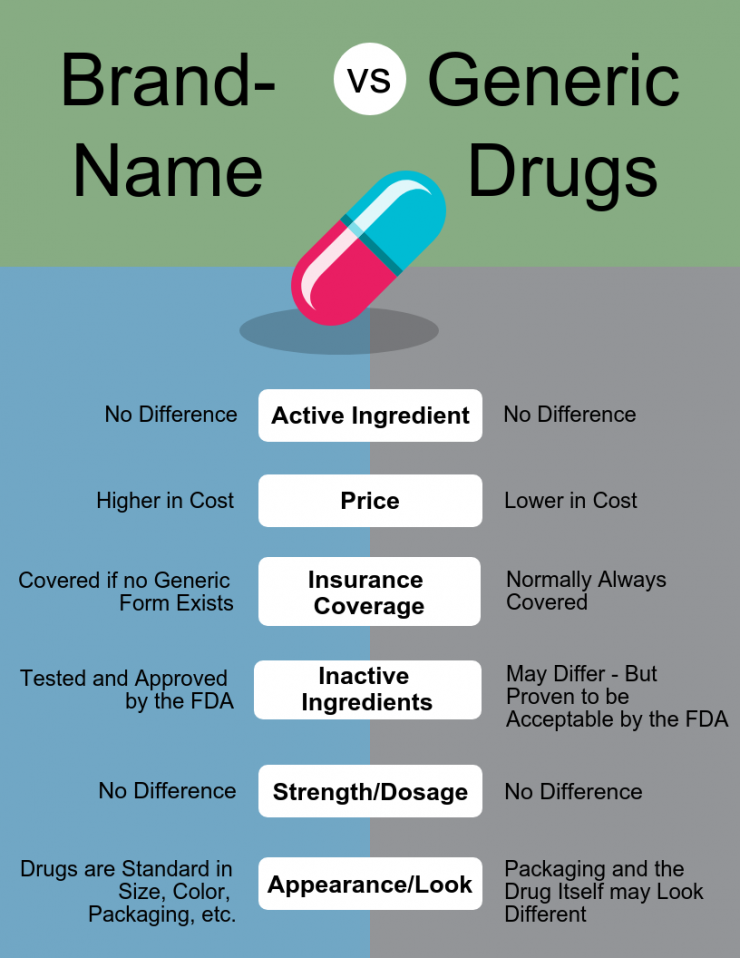
Difference Between Generic And Hybrid Medicine . a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we compared generic vs. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european.
from hxedegqfb.blob.core.windows.net
a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. we compared generic vs. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine.
What Is The Difference Between Generic Medicine And Other Medicine at
Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we compared generic vs. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain.
From joinhubpharma.com
What's the Difference? Biosimilar and Generic Drugs JoinHub Pharma Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine.. Difference Between Generic And Hybrid Medicine.
From hxefubrre.blob.core.windows.net
Difference Between Generic And Regular Medicine at Stella Conrad blog Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. . Difference Between Generic And Hybrid Medicine.
From emergencydrug.com
Generic medication Archives Emergency Drug Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid. Difference Between Generic And Hybrid Medicine.
From clickserre.weebly.com
Symbicort copay card clickserre Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us. Difference Between Generic And Hybrid Medicine.
From hxedegqfb.blob.core.windows.net
What Is The Difference Between Generic Medicine And Other Medicine at Difference Between Generic And Hybrid Medicine we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday.. Difference Between Generic And Hybrid Medicine.
From giobmmpka.blob.core.windows.net
Name Medicine Price at Raymond Bush blog Difference Between Generic And Hybrid Medicine a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. . Difference Between Generic And Hybrid Medicine.
From xeteor.com
Brand vs. Generic Drugs What's the Difference? Difference Between Generic And Hybrid Medicine a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. hybrid. Difference Between Generic And Hybrid Medicine.
From jnccn.org
The Role of Biosimilars in Journal of the National Comprehensive Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us. Difference Between Generic And Hybrid Medicine.
From www.bharatagritech.com
Difference Between Biologics And Biosimilars Compare The, 55 OFF Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the. Difference Between Generic And Hybrid Medicine.
From exocatfoo.blob.core.windows.net
Difference Between Generic And Original Medicine at Cruz Freeman blog Difference Between Generic And Hybrid Medicine a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined. Difference Between Generic And Hybrid Medicine.
From www.keyscriptsllc.com
Brand vs. Generic Drugs Q&A Part One of a ThreePart Series Difference Between Generic And Hybrid Medicine we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us food and drug administration (fda) and the european medicines agency. Difference Between Generic And Hybrid Medicine.
From www.slideserve.com
PPT Introduction to Biosimilars PowerPoint Presentation ID6891333 Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we compared generic vs. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european.. Difference Between Generic And Hybrid Medicine.
From thinkpharmacy.com.au
Any Difference Between Generic And Branded Drugs? Think Pharmacy Difference Between Generic And Hybrid Medicine a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. we compared generic. Difference Between Generic And Hybrid Medicine.
From www.nicerx.com
Biosimilar vs generic which is better? NiceRx Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday.. Difference Between Generic And Hybrid Medicine.
From exocatfoo.blob.core.windows.net
Difference Between Generic And Original Medicine at Cruz Freeman blog Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. we compared generic vs. a. Difference Between Generic And Hybrid Medicine.
From www.pyxuspharmaceuticals.com
Difference Between the Branded Drugs and Generic Drugs Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. . Difference Between Generic And Hybrid Medicine.
From exocatfoo.blob.core.windows.net
Difference Between Generic And Original Medicine at Cruz Freeman blog Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we. Difference Between Generic And Hybrid Medicine.
From www.researchgate.net
Differences between Generics and Biosimilars Generics Biosimilars Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. we compared generic vs. a medicine that is similar to an authorised medicine containing the same active substance, but where there are. Difference Between Generic And Hybrid Medicine.
From discover.hubpages.com
What's the Difference Between Generic and BrandName Medicine? HubPages Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we compared generic vs. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a. Difference Between Generic And Hybrid Medicine.
From exocatfoo.blob.core.windows.net
Difference Between Generic And Original Medicine at Cruz Freeman blog Difference Between Generic And Hybrid Medicine a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. a generic. Difference Between Generic And Hybrid Medicine.
From www.sketchbubble.com
Generic Drugs Vs Branded Drugs PowerPoint and Google Slides Template Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised,. Difference Between Generic And Hybrid Medicine.
From www.youtube.com
Original Vs Generic Drugs. Is there a difference? Which is better Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a. Difference Between Generic And Hybrid Medicine.
From www.collidu.com
Generic Drugs Vs Branded Drugs PowerPoint and Google Slides Template Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. we compared generic vs. a medicine that is similar to an authorised medicine containing the same active substance, but where there. Difference Between Generic And Hybrid Medicine.
From hxefubrre.blob.core.windows.net
Difference Between Generic And Regular Medicine at Stella Conrad blog Difference Between Generic And Hybrid Medicine we compared generic vs. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday.. Difference Between Generic And Hybrid Medicine.
From vetmed.illinois.edu
Pharmacist’s Corner Biosimilars and Generic Drugs Veterinary Difference Between Generic And Hybrid Medicine we compared generic vs. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. a generic medicine is developed to be the same as a medicine that has already been authorised, called. Difference Between Generic And Hybrid Medicine.
From www.mdedge.com
Cost gap widens between brandname, generic drugs MDedge Internal Difference Between Generic And Hybrid Medicine we compared generic vs. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in. Difference Between Generic And Hybrid Medicine.
From www.semanticscholar.org
[PDF] A Review on Generic and Branded of Generic Drugs Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. the european medicines. Difference Between Generic And Hybrid Medicine.
From www.doctor-4-u.co.uk
Branded vs Generic Medication What’s the Difference? Doctor4U Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to. Difference Between Generic And Hybrid Medicine.
From www.semanticscholar.org
Figure 1 from Understanding Key Differences Between Biosimilars and Difference Between Generic And Hybrid Medicine a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. we compared generic vs. a generic medicine is developed to be the same as a medicine that has. Difference Between Generic And Hybrid Medicine.
From www.youtube.com
Difference between generic and branded medicine YouTube Difference Between Generic And Hybrid Medicine a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. hybrid medicines are defined as medicines. Difference Between Generic And Hybrid Medicine.
From rxbiotech.in
What is the difference between generic and ethical medicine? Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. we compared. Difference Between Generic And Hybrid Medicine.
From canada.altasciences.com
Will Biosimilars Replace Biologics? Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. we compared generic vs. a. Difference Between Generic And Hybrid Medicine.
From mybiologydictionary.com
Difference between Cybrids and Hybrids in Plant Biotechnology My Difference Between Generic And Hybrid Medicine the us food and drug administration (fda) and the european medicines agency (ema) on wednesday. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. we compared generic vs. hybrid. Difference Between Generic And Hybrid Medicine.
From www.insightsonindia.com
Drugs and Cosmetic Rules, 1945 INSIGHTS IAS Simplifying UPSC IAS Difference Between Generic And Hybrid Medicine hybrid medicines are defined as medicines that do not fill generic medicines' definition, forming a medicines group that stands. the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a medicine that is similar to an authorised medicine containing the same active substance, but where there are certain. the us. Difference Between Generic And Hybrid Medicine.
From healthmatch.io
HealthMatch Are Generic Drugs Just As Good As Branded Drugs? Difference Between Generic And Hybrid Medicine the european medicines agency (ema) assesses applications from companies to market generic medicines in the european. a generic medicine is developed to be the same as a medicine that has already been authorised, called the reference medicine. we compared generic vs. the us food and drug administration (fda) and the european medicines agency (ema) on wednesday.. Difference Between Generic And Hybrid Medicine.