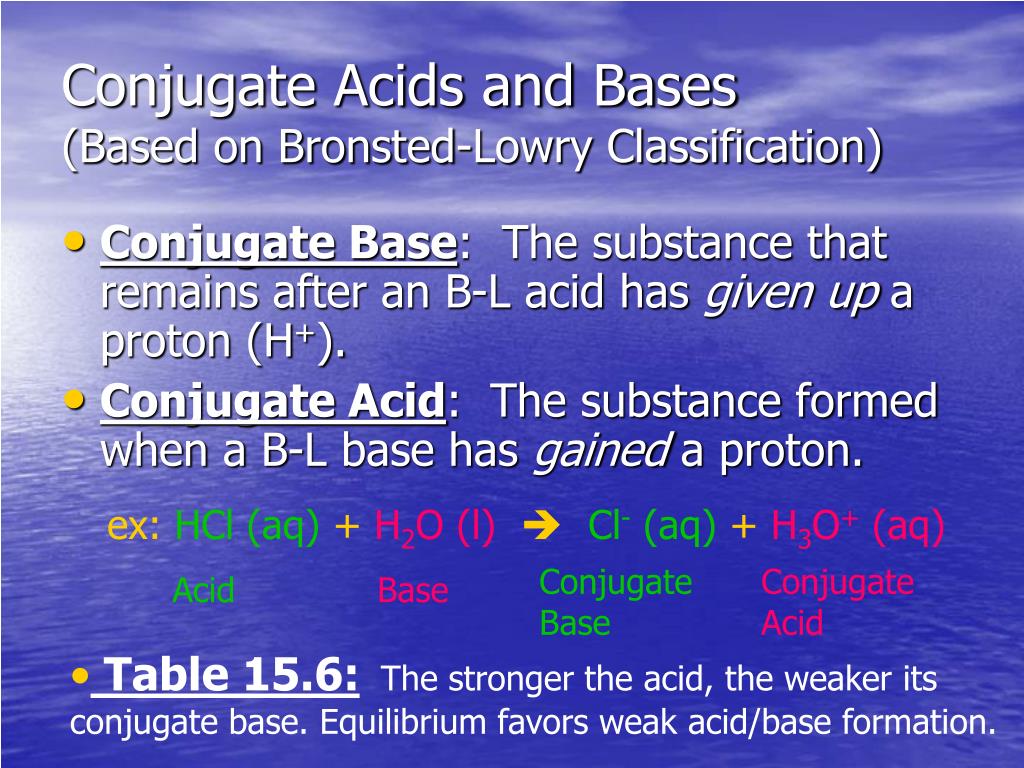
Bromine Conjugate Base . conjugate acids and bases. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). the key to understanding this trend is to consider the hypothetical conjugate base in each case: to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. One important consequence of these equilibria is that every acid (ha) has a. The acid and base chart is a. The more stable (weaker) the. When this acid donates an h + ion to water, one. Imagine a generic acid, ha.
from www.slideserve.com
Imagine a generic acid, ha. The more stable (weaker) the. the key to understanding this trend is to consider the hypothetical conjugate base in each case: conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. One important consequence of these equilibria is that every acid (ha) has a. When this acid donates an h + ion to water, one. The acid and base chart is a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1).
PPT Acids and Bases PowerPoint Presentation, free download ID3516179
Bromine Conjugate Base When this acid donates an h + ion to water, one. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). conjugate acids and bases. Imagine a generic acid, ha. The acid and base chart is a. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. When this acid donates an h + ion to water, one. the key to understanding this trend is to consider the hypothetical conjugate base in each case: The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a.
From study.com
How to Determine Conjugate Acid or Base Strength Chemistry Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). Imagine a generic acid, ha. When this acid donates an h + ion to water, one. The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a. the key to understanding. Bromine Conjugate Base.
From www.slideserve.com
PPT Br ø nstedLowry and Lewis Acids/Bases PowerPoint Presentation Bromine Conjugate Base the key to understanding this trend is to consider the hypothetical conjugate base in each case: When this acid donates an h + ion to water, one. Imagine a generic acid, ha. The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a. to compare the acidic strengths of the haloforms,. Bromine Conjugate Base.
From www.studypool.com
SOLUTION Conjugate Acid and Conjugate Base pKa Chart Studypool Bromine Conjugate Base the key to understanding this trend is to consider the hypothetical conjugate base in each case: to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. One important consequence of these equilibria is that every acid (ha) has a. Imagine a generic acid, ha. the \(no_3−\) anion is. Bromine Conjugate Base.
From www.slideserve.com
PPT I. Introduction to Acids & Bases PowerPoint Presentation ID5642124 Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). Imagine a generic acid, ha. When this acid donates an h + ion to water, one. One important consequence of these equilibria is that every acid (ha) has a. The more stable (weaker) the. The acid and base chart. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2948655 Bromine Conjugate Base When this acid donates an h + ion to water, one. One important consequence of these equilibria is that every acid (ha) has a. conjugate acids and bases. The acid and base chart is a. the key to understanding this trend is to consider the hypothetical conjugate base in each case: Imagine a generic acid, ha. the. Bromine Conjugate Base.
From www.slideserve.com
PPT To understand two models of acids and bases PowerPoint Bromine Conjugate Base to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The more stable (weaker) the. conjugate acids and bases. The acid and base chart is a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this. Bromine Conjugate Base.
From thechemistrynotes.com
Bromine (Br) Element Properties and Amazing Uses, Facts Bromine Conjugate Base conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The acid and base chart is a. The more stable (weaker) the. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this. Bromine Conjugate Base.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Bromine Conjugate Base to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a. Imagine a generic acid, ha. The acid and base chart is a. the \(no_3−\) anion is the conjugate base of a. Bromine Conjugate Base.
From www.slideserve.com
PPT Acid and Bases An Introduction PowerPoint Presentation, free Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. conjugate acids and bases. One important consequence of these equilibria is that every acid (ha) has a. . Bromine Conjugate Base.
From www.masterorganicchemistry.com
Halogenation Of Ketones via Enols Master Organic Chemistry Bromine Conjugate Base conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. the key to understanding this trend is to consider the hypothetical conjugate base in each case: One important consequence of these equilibria is that every acid (ha) has a. The more stable (weaker) the.. Bromine Conjugate Base.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Bromine Conjugate Base the key to understanding this trend is to consider the hypothetical conjugate base in each case: to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a. When this acid donates an. Bromine Conjugate Base.
From slideplayer.com
Chapter 9 Acids and Bases ppt download Bromine Conjugate Base When this acid donates an h + ion to water, one. The acid and base chart is a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). Imagine a generic acid, ha. the key to understanding this trend is to consider the hypothetical conjugate base in each. Bromine Conjugate Base.
From www.slideserve.com
PPT Br ø nstedLowry and Lewis Acids/Bases PowerPoint Presentation Bromine Conjugate Base When this acid donates an h + ion to water, one. The acid and base chart is a. the key to understanding this trend is to consider the hypothetical conjugate base in each case: Imagine a generic acid, ha. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases.. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids PowerPoint Presentation, free download ID5970481 Bromine Conjugate Base The more stable (weaker) the. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). The acid and base chart is a. One important consequence of these equilibria is that every acid (ha) has a. Imagine a generic acid, ha. conjugate acids and bases. to compare the. Bromine Conjugate Base.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Conjugate Base the key to understanding this trend is to consider the hypothetical conjugate base in each case: One important consequence of these equilibria is that every acid (ha) has a. The acid and base chart is a. The more stable (weaker) the. conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check. Bromine Conjugate Base.
From www.chegg.com
Solved For the Brønsted acidbase reaction shown in the Bromine Conjugate Base The more stable (weaker) the. One important consequence of these equilibria is that every acid (ha) has a. conjugate acids and bases. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). the key to understanding this trend is to consider the hypothetical conjugate base in each. Bromine Conjugate Base.
From www.slideserve.com
PPT Strengths of Conjugate AcidBase Pairs PowerPoint Presentation Bromine Conjugate Base One important consequence of these equilibria is that every acid (ha) has a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this acid donates an h + ion to water, one. The more stable (weaker) the. Imagine a generic acid, ha. the key to understanding. Bromine Conjugate Base.
From slideplayer.com
Intro to Acids & Bases. ppt download Bromine Conjugate Base One important consequence of these equilibria is that every acid (ha) has a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). the key to understanding this trend is to consider the hypothetical conjugate base in each case: to compare the acidic strengths of the haloforms,. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID3516179 Bromine Conjugate Base Imagine a generic acid, ha. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). the key to understanding this trend is to consider the hypothetical conjugate base in each case: to compare the acidic strengths of the haloforms, we need to check the relative stabilities of. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID1483399 Bromine Conjugate Base The more stable (weaker) the. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The acid and base chart is a. One important consequence of these equilibria is that every acid (ha) has a. When this acid donates an h + ion to water, one. the \(no_3−\) anion. Bromine Conjugate Base.
From www.slideserve.com
PPT Acid and Base PowerPoint Presentation, free download ID6399344 Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). The acid and base chart is a. When this acid donates an h + ion to water, one. conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check the relative stabilities. Bromine Conjugate Base.
From www.youtube.com
Conjugate Acids and Bases Chapter 16 Part 2 YouTube Bromine Conjugate Base Imagine a generic acid, ha. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The acid and base chart is a. One important consequence of these equilibria is that every acid (ha) has a. conjugate acids and bases. When this acid donates an h + ion to water,. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID969733 Bromine Conjugate Base The more stable (weaker) the. The acid and base chart is a. Imagine a generic acid, ha. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. When this acid donates an h + ion to water, one. the \(no_3−\) anion is the conjugate base of a strong acid,. Bromine Conjugate Base.
From slideplayer.com
I. Introduction to Acids & Bases ppt download Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). conjugate acids and bases. Imagine a generic acid, ha. The more stable (weaker) the. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. the key to. Bromine Conjugate Base.
From www.slideserve.com
PPT Br ø nstedLowry and Lewis Acids/Bases PowerPoint Presentation Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). The more stable (weaker) the. conjugate acids and bases. The acid and base chart is a. When this acid donates an h + ion to water, one. Imagine a generic acid, ha. to compare the acidic strengths. Bromine Conjugate Base.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Bromine Conjugate Base One important consequence of these equilibria is that every acid (ha) has a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this acid donates an h + ion to water, one. The acid and base chart is a. Imagine a generic acid, ha. the key. Bromine Conjugate Base.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID6155898 Bromine Conjugate Base When this acid donates an h + ion to water, one. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. The acid and base chart is a. conjugate acids and bases. the key to understanding this trend is to consider the hypothetical conjugate base in each case:. Bromine Conjugate Base.
From kunduz.com
[ANSWERED] Turn this molecule into its Br nsted Lowry conjugate base Bromine Conjugate Base Imagine a generic acid, ha. The acid and base chart is a. When this acid donates an h + ion to water, one. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no. Bromine Conjugate Base.
From www.youtube.com
Recognizing relative strength of conjugate acids and bases YouTube Bromine Conjugate Base One important consequence of these equilibria is that every acid (ha) has a. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). Imagine a generic acid, ha. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. When. Bromine Conjugate Base.
From ar.inspiredpencil.com
H2so4 Lewis Structure Conjugate Base Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this acid donates an h + ion to water, one. conjugate acids and bases. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. the key. Bromine Conjugate Base.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Bromine Conjugate Base conjugate acids and bases. the key to understanding this trend is to consider the hypothetical conjugate base in each case: Imagine a generic acid, ha. The more stable (weaker) the. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this acid donates an h +. Bromine Conjugate Base.
From www.toppr.com
The conjugate base of the weak acid in the reaction HBr + H2O → H3O Bromine Conjugate Base The more stable (weaker) the. the key to understanding this trend is to consider the hypothetical conjugate base in each case: Imagine a generic acid, ha. One important consequence of these equilibria is that every acid (ha) has a. The acid and base chart is a. conjugate acids and bases. When this acid donates an h + ion. Bromine Conjugate Base.
From www.numerade.com
SOLVED Draw the structure of a ring of seven carbons with three Bromine Conjugate Base the key to understanding this trend is to consider the hypothetical conjugate base in each case: One important consequence of these equilibria is that every acid (ha) has a. The acid and base chart is a. The more stable (weaker) the. the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic. Bromine Conjugate Base.
From www.numerade.com
SOLVED Draw the conjugate base for each acid, including all lone pairs Bromine Conjugate Base the \(no_3−\) anion is the conjugate base of a strong acid, so it has essentially no basic character (table 16.1). When this acid donates an h + ion to water, one. Imagine a generic acid, ha. The more stable (weaker) the. to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their. Bromine Conjugate Base.
From www.slideserve.com
PPT Br ø nstedLowry and Lewis Acids/Bases PowerPoint Presentation Bromine Conjugate Base to compare the acidic strengths of the haloforms, we need to check the relative stabilities of their conjugate bases. When this acid donates an h + ion to water, one. The acid and base chart is a. One important consequence of these equilibria is that every acid (ha) has a. The more stable (weaker) the. the \(no_3−\) anion. Bromine Conjugate Base.