Zinc Carbonate Decomposes When Heated . When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. Therefore, the chemical equation that shows the thermal. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The alkaline earth metal carbonates. Therefore, the chemical equation that shows the thermal. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The carbonates become more stable to heat as you go down the group. All the nitrates in this group undergo thermal decomposition to give the. The effect of heat on the group 2 nitrates. Copper carbonate decomposes at 290 °c. What is the thermal decomposition of carbonates? Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach.
from www.numerade.com
Therefore, the chemical equation that shows the thermal. The effect of heat on the group 2 nitrates. The alkaline earth metal carbonates. The carbonates become more stable to heat as you go down the group. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. What is the thermal decomposition of carbonates? Cuco3 (s) → cuo (s) + co2 (g) examiner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner.
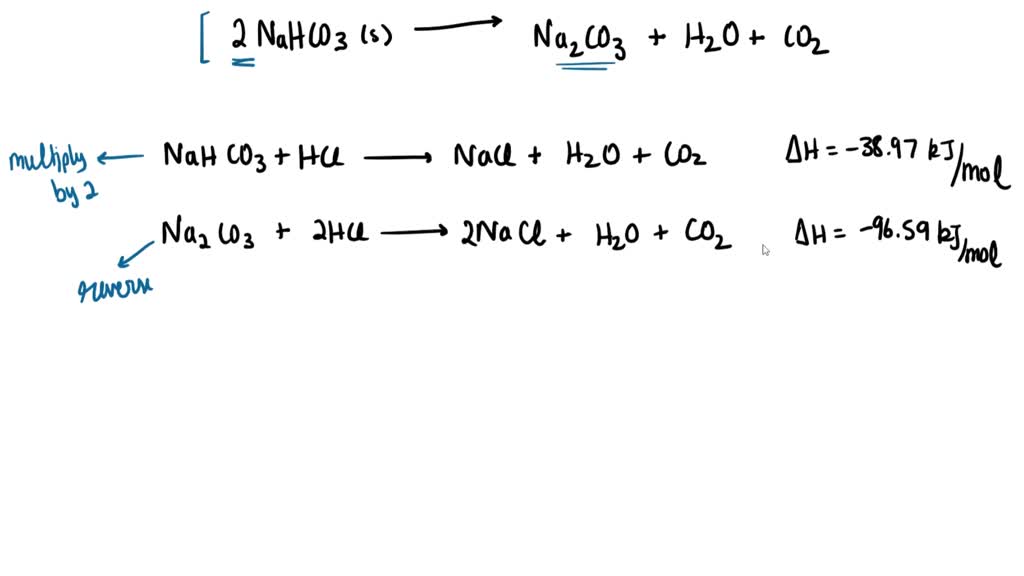
SOLVED 1)Baking soda (NaHCO3) on heating as follows 2
Zinc Carbonate Decomposes When Heated Copper carbonate decomposes at 290 °c. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Therefore, the chemical equation that shows the thermal. Therefore, the chemical equation that shows the thermal. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The effect of heat on the group 2 nitrates. Copper carbonate decomposes at 290 °c. All the nitrates in this group undergo thermal decomposition to give the. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Cuco3 (s) → cuo (s) + co2 (g) examiner. What is the thermal decomposition of carbonates? Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The carbonates become more stable to heat as you go down the group.
From www.chegg.com
Solved 1. Lithium hydrogen carbonate by heating Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. The effect of heat on the group 2 nitrates. The carbonates become more stable to heat as you go down the group. The alkaline earth metal carbonates. Copper carbonate decomposes at 290 °c. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. The group. Zinc Carbonate Decomposes When Heated.
From www.slideshare.net
Action of heat on chemical compound Zinc Carbonate Decomposes When Heated When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. Therefore, the chemical equation that shows the thermal. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Therefore, the chemical equation that shows the thermal. Thermal decomposition can occur for many different. Zinc Carbonate Decomposes When Heated.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Carbonate Decomposes When Heated Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. Cuco3 (s) → cuo (s) + co2 (g) examiner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The carbonates become more stable to heat as you go down the group. Copper carbonate decomposes. Zinc Carbonate Decomposes When Heated.
From www.numerade.com
Magnesium carbonate into magnesium Zinc Carbonate Decomposes When Heated Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Therefore, the chemical. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved When heated, calcium carbonate t calcium Zinc Carbonate Decomposes When Heated The alkaline earth metal carbonates. Cuco3 (s) → cuo (s) + co2 (g) examiner. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal. Zinc Carbonate Decomposes When Heated.
From www.glochem.com
Comprehensive Guide to Zinc Carbonate Properties, Applications, and Zinc Carbonate Decomposes When Heated Therefore, the chemical equation that shows the thermal. The alkaline earth metal carbonates. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are. Zinc Carbonate Decomposes When Heated.
From www.toppr.com
(iv) A nonmetal of valency 4. (v) An element with 6 electrons in in Zinc Carbonate Decomposes When Heated What is the thermal decomposition of carbonates? Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The carbonates become more stable to heat as. Zinc Carbonate Decomposes When Heated.
From www.coursehero.com
[Solved] 1. Lithium hydrogen carbonate by heating as shown Zinc Carbonate Decomposes When Heated For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Therefore, the chemical equation that shows the thermal. Cuco3 (s) → cuo (s) + co2 (g) examiner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. Copper carbonate decomposes at 290 °c.. Zinc Carbonate Decomposes When Heated.
From www.numerade.com
SOLVED 1)Baking soda (NaHCO3) on heating as follows 2 Zinc Carbonate Decomposes When Heated For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Thermal. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved When heated, calcium carbonate to yield Zinc Carbonate Decomposes When Heated All the nitrates in this group undergo thermal decomposition to give the. What is the thermal decomposition of carbonates? The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Therefore, the chemical equation that shows the thermal. The effect of heat on the group 2 nitrates. Cuco3 (s). Zinc Carbonate Decomposes When Heated.
From www.youtube.com
Heating zinc carbonate YouTube Zinc Carbonate Decomposes When Heated The effect of heat on the group 2 nitrates. Copper carbonate decomposes at 290 °c. The carbonates become more stable to heat as you go down the group. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner.. Zinc Carbonate Decomposes When Heated.
From byjus.com
20 g of an impure sample of calcium carbonate on heating to Zinc Carbonate Decomposes When Heated All the nitrates in this group undergo thermal decomposition to give the. Therefore, the chemical equation that shows the thermal. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. When zinc carbonate is heated, it will decompose to produce. Zinc Carbonate Decomposes When Heated.
From www.youtube.com
ACTION OF HEAT ON ZINC CARBONATE YouTube Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. The carbonates become more stable to heat as you go down the group. The alkaline earth metal carbonates. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The effect of heat on the group 2 nitrates.. Zinc Carbonate Decomposes When Heated.
From www.numerade.com
SOLVED 16. Write the following in the form of balanced chemical Zinc Carbonate Decomposes When Heated The alkaline earth metal carbonates. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The carbonates become more stable to heat as you go down the group. What is the thermal decomposition of carbonates? When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas.. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved When heated, calcium carbonate to yield Zinc Carbonate Decomposes When Heated Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. All the nitrates in this group undergo thermal decomposition to give the. Therefore, the chemical equation that shows the thermal. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The group. Zinc Carbonate Decomposes When Heated.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Carbonate Decomposes When Heated For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Therefore, the chemical equation that shows the thermal. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon. Zinc Carbonate Decomposes When Heated.
From www.toppr.com
IB, May 2006 7 Calcium carbonate on heating as shown below Zinc Carbonate Decomposes When Heated What is the thermal decomposition of carbonates? The carbonates become more stable to heat as you go down the group. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Copper carbonate decomposes. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved Part A When heated, calcium carbonate to Zinc Carbonate Decomposes When Heated The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The effect of heat on the group 2 nitrates. Copper carbonate decomposes at 290 °c. For example, copper, lead, and zinc carbonates all break. Zinc Carbonate Decomposes When Heated.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Decomposes When Heated All the nitrates in this group undergo thermal decomposition to give the. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The carbonates become more stable to heat as you go down the group. Cuco3 (s) → cuo (s) + co2 (g) examiner. Compare the thermal stabilities of. Zinc Carbonate Decomposes When Heated.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.3.4 Carbon Dioxide from Zinc Carbonate Decomposes When Heated When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The effect of heat on the group 2 nitrates. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. For example, copper, lead, and zinc carbonates all. Zinc Carbonate Decomposes When Heated.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Zinc Carbonate Decomposes When Heated The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Cuco3 (s) → cuo (s) + co2 (g) examiner. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which. Zinc Carbonate Decomposes When Heated.
From www.youtube.com
20 g of magnesium carbonate sample on heating to give carbon Zinc Carbonate Decomposes When Heated The alkaline earth metal carbonates. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. What is the thermal decomposition of carbonates? Therefore, the chemical equation that shows the thermal. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. When zinc. Zinc Carbonate Decomposes When Heated.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate Decomposes When Heated Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Therefore, the chemical equation that shows the thermal. Therefore, the chemical equation that shows the thermal. Compare the thermal stabilities of carbonates of reactive. Zinc Carbonate Decomposes When Heated.
From www.shutterstock.com
Reaction Infographic Diagram Example Zinc Stock Vector Zinc Carbonate Decomposes When Heated The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Therefore, the chemical equation that shows the thermal. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The effect of heat on the. Zinc Carbonate Decomposes When Heated.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Decomposes When Heated Copper carbonate decomposes at 290 °c. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The effect of heat on the group 2 nitrates. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The carbonates become more stable to heat as you go. Zinc Carbonate Decomposes When Heated.
From brainly.in
2.5g zinc carbonare is heated what is the mass of zinc oxide formed Zinc Carbonate Decomposes When Heated Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. All the nitrates in this group undergo thermal. Zinc Carbonate Decomposes When Heated.
From hxeopfikj.blob.core.windows.net
Zinc Carbonate Is Heated Equation at Virgie Lynn blog Zinc Carbonate Decomposes When Heated The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. The carbonates become more stable to heat as you go down the group. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. When. Zinc Carbonate Decomposes When Heated.
From www.numerade.com
SOLVED When heated, calcium carbonate to yield calcium Zinc Carbonate Decomposes When Heated The alkaline earth metal carbonates. All the nitrates in this group undergo thermal decomposition to give the. The carbonates become more stable to heat as you go down the group. What is the thermal decomposition of carbonates? For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. The group 2 carbonates break down (decompose). Zinc Carbonate Decomposes When Heated.
From www.numerade.com
12.5g of zinc carbonate is heated, it to make 8.1g of zinc Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. For example, copper, lead, and zinc carbonates all break down when heated with a bunsen burner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved Calcium carbonate when heated to solid Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. What is the thermal decomposition of carbonates? The carbonates become more stable to heat as you go down the group. All the nitrates in this group undergo thermal decomposition to give the. Therefore, the chemical equation that shows the thermal. Therefore, the chemical equation that shows the thermal. The effect of. Zinc Carbonate Decomposes When Heated.
From byjus.com
20.0 g of a magnesium carbonate sample on heating to give Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The carbonates become more stable to heat as you go down the group. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Therefore,. Zinc Carbonate Decomposes When Heated.
From mccnsulting.web.fc2.com
What happens when lead nitrate is heated? Zinc Carbonate Decomposes When Heated Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Therefore, the chemical equation that shows the thermal. The carbonates become more stable to heat as you go down the group. When. Zinc Carbonate Decomposes When Heated.
From www.toppr.com
IB, MU poses on heating as 4 Calcium carbonate on heatin Zinc Carbonate Decomposes When Heated Cuco3 (s) → cuo (s) + co2 (g) examiner. Copper carbonate decomposes at 290 °c. All the nitrates in this group undergo thermal decomposition to give the. What is the thermal decomposition of carbonates? Thermal decomposition can occur for many different carbonates, but not for all metal carbonates. The effect of heat on the group 2 nitrates. When zinc carbonate. Zinc Carbonate Decomposes When Heated.
From www.slideserve.com
PPT The limestone cycle PowerPoint Presentation, free download ID Zinc Carbonate Decomposes When Heated Therefore, the chemical equation that shows the thermal. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The effect of heat on the group 2 nitrates. The alkaline earth metal carbonates. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner. Zinc Carbonate Decomposes When Heated.
From www.chegg.com
Solved 1. Lithium hydrogen carbonate by heating Zinc Carbonate Decomposes When Heated The alkaline earth metal carbonates. Therefore, the chemical equation that shows the thermal. When zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off carbon dioxide gas. Copper, zinc and calcium. Zinc Carbonate Decomposes When Heated.