Medical Device Design Validation . Design verification and validation are two essential steps in medical device product development. As part of design and development validation, the organization shall perform clinical evaluations or performance. Medical device validation determines whether you built the right product for its intended use. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. What are medical device companies requesting these days when it comes to validation and verification? Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. With this guide, i plan to share valuable insights to explain what design. It’s easy to confuse the two, but there’s a simple way to remember them: Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Learn the benefits and key steps of medical device validation.
from beacon-india.com
With this guide, i plan to share valuable insights to explain what design. Design verification and validation are two essential steps in medical device product development. Medical device validation determines whether you built the right product for its intended use. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. It’s easy to confuse the two, but there’s a simple way to remember them: As part of design and development validation, the organization shall perform clinical evaluations or performance. What are medical device companies requesting these days when it comes to validation and verification? Learn the benefits and key steps of medical device validation.
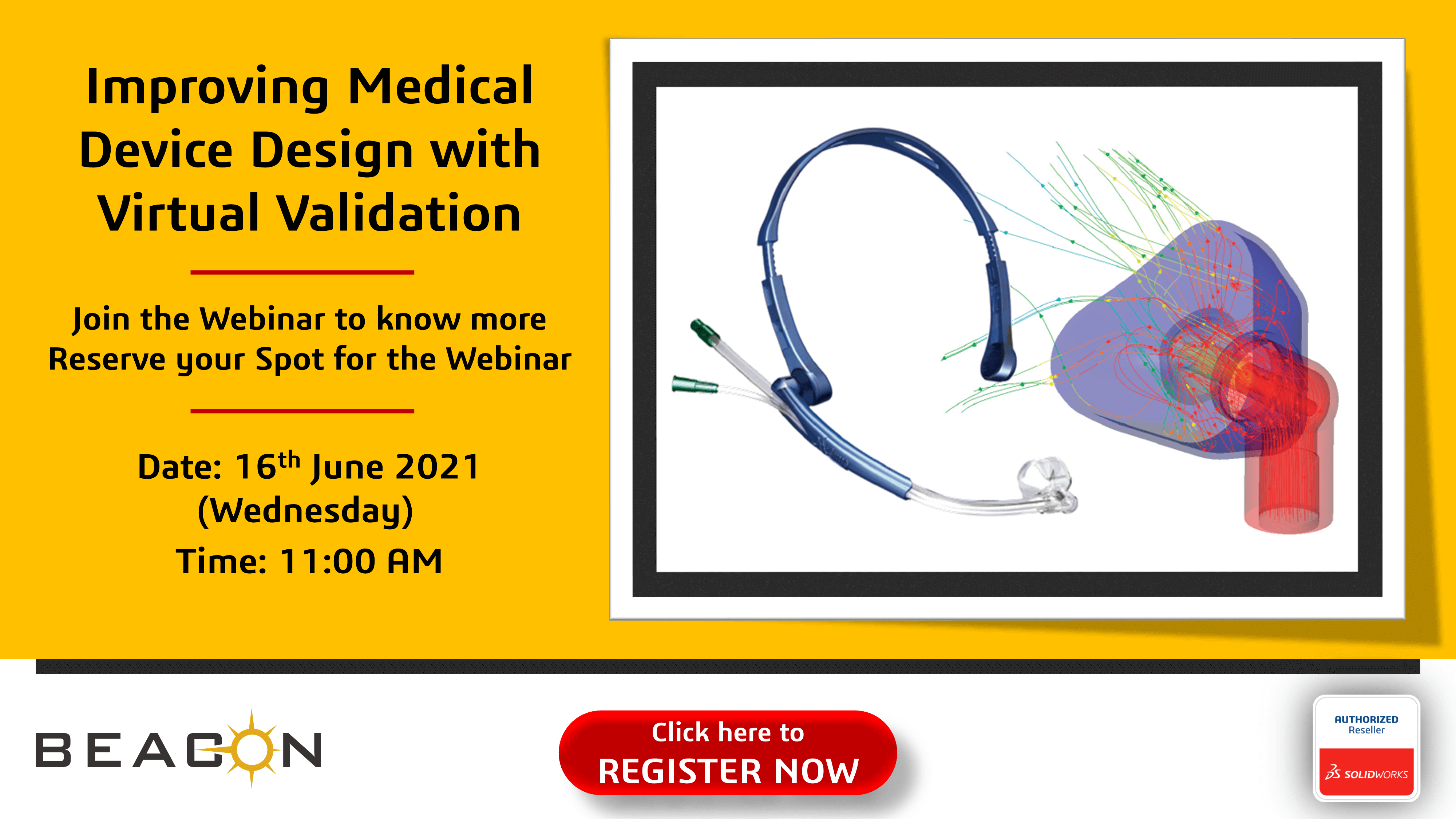
Improving Medical Device Design with Virtual Validation BEACON INDIA
Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. Design verification and validation are two essential steps in medical device product development. It’s easy to confuse the two, but there’s a simple way to remember them: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Learn the benefits and key steps of medical device validation. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. What are medical device companies requesting these days when it comes to validation and verification? As part of design and development validation, the organization shall perform clinical evaluations or performance. With this guide, i plan to share valuable insights to explain what design. Medical device validation determines whether you built the right product for its intended use.
From flamlabelthema.netlify.app
Medical Device Design Validation Protocol Template Medical Device Design Validation It’s easy to confuse the two, but there’s a simple way to remember them: With this guide, i plan to share valuable insights to explain what design. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Design verification and validation are two essential steps in medical device product development. What are medical device companies. Medical Device Design Validation.
From www.alecalpert.com
Medical Device Design Verification Essentials Alec Alpert Medical Device Design Validation It’s easy to confuse the two, but there’s a simple way to remember them: Learn the benefits and key steps of medical device validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. Medical device validation determines whether you built the right product for its intended use. Design controls demonstrate our medical devices are. Medical Device Design Validation.
From www.scribd.com
Medical Device Design Validation SOP Verification And Validation Medical Device Design Validation Learn the benefits and key steps of medical device validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use.. Medical Device Design Validation.
From www.padtinc.com
FDA Opening to Simulation Supported Verification and Validation for Medical Device Design Validation Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Learn the benefits and key steps of medical device validation. It’s easy to confuse the two, but there’s a simple way to remember them: If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. As part. Medical Device Design Validation.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? Learn the benefits and key steps of medical device validation. Medical device validation determines whether you built the right product for its intended use. Design verification and validation are two essential steps in medical device product development. If you develop products — medical devices, particularly. Medical Device Design Validation.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Design Validation Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are medical device companies requesting these days when it comes to validation and verification? Learn the benefits and key steps of medical device validation. It’s easy to confuse the two, but there’s a simple way to remember them: Medical. Medical Device Design Validation.
From data1.skinnyms.com
Medical Device Verification And Validation Plan Template Medical Device Design Validation Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Learn the benefits and key steps of medical device validation. With this guide, i plan to share valuable insights to explain what design. Medical device validation determines whether you built the right product for its intended use. What are medical device companies requesting these days. Medical Device Design Validation.
From arrotek.com
Medical Device Design Validation Explained Arrotek Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. It’s easy to confuse the two, but there’s a simple way to remember them: As part of design and development validation, the organization shall perform clinical evaluations or performance. Design verification and validation are two essential steps in medical device product development. What are medical device. Medical Device Design Validation.
From www.cognidox.com
Design verification & design validation for medical device developers Medical Device Design Validation Learn the benefits and key steps of medical device validation. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design verification and validation are two essential steps in medical device product development. What are medical device companies requesting these days when it comes to validation and verification? Safe medical device. Medical Device Design Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? As part of design and development validation, the organization shall perform clinical evaluations or performance. It’s easy to confuse the two, but there’s a simple way to remember them: Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Medical. Medical Device Design Validation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Design Validation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Learn the benefits and key steps of medical device validation. Safe medical device act of 1990 authorized fda to add design controls to the current good. Medical Device Design Validation.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Design Validation Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. What are medical device companies requesting these days when it comes to validation and verification? It’s easy to confuse the two, but there’s a simple way to remember them: Learn the benefits and key steps of medical device validation. If you develop products — medical. Medical Device Design Validation.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Validation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. It’s easy to confuse the two, but there’s a simple way to remember them: What are medical device companies requesting these days when it comes to validation and verification? As part of design and development validation, the organization shall perform clinical. Medical Device Design Validation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Design Validation It’s easy to confuse the two, but there’s a simple way to remember them: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. What are medical device companies requesting these days when it comes to validation and verification? As part of design and development validation, the organization shall perform. Medical Device Design Validation.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Design Validation Design verification and validation are two essential steps in medical device product development. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. As part of design and development validation, the organization shall perform clinical evaluations or performance. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing. Medical Device Design Validation.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Medical Device Design Validation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Learn the benefits and key steps of medical device validation. It’s easy to confuse the two, but there’s a simple way to remember them: Design verification and validation are two essential steps in medical device product development. What are medical device. Medical Device Design Validation.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? It’s easy to confuse the two, but there’s a simple way to remember them: Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Medical device validation determines whether you built the right product for its intended use. Learn the. Medical Device Design Validation.
From beacon-india.com
Improving Medical Device Design with Virtual Validation BEACON INDIA Medical Device Design Validation Design verification and validation are two essential steps in medical device product development. It’s easy to confuse the two, but there’s a simple way to remember them: Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice. Medical Device Design Validation.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development Medical Device Design Validation Learn the benefits and key steps of medical device validation. Design verification and validation are two essential steps in medical device product development. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Medical device validation determines whether you built the right product for its intended use. It’s easy to. Medical Device Design Validation.
From www.slideteam.net
Medical Device Process Validation Flowchart Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. What are medical device companies requesting these days when it comes to validation and verification? It’s easy to confuse the two, but there’s a simple way to remember them: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing. Medical Device Design Validation.
From qaconsultinginc.com
Design Verification vs. Design Validation What’s The Difference? QA Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? Design verification and validation are two essential steps in medical device product development. Medical device validation determines whether you built the right product for its intended use. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device. Medical Device Design Validation.
From tsqasia.com
Medical Devices EU Design Control Process Support TSQ Middle East & Asia Medical Device Design Validation Learn the benefits and key steps of medical device validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design verification and validation are two essential steps in medical device product development. Medical device validation. Medical Device Design Validation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. As part of design and development validation, the organization shall perform clinical evaluations or performance. It’s easy to confuse the two, but there’s a simple way to remember them: What are medical device companies requesting these days when it comes to validation and verification? Safe medical. Medical Device Design Validation.
From www.greenlight.guru
Medical Device Design Verification Greenlight Guru Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. Learn the benefits and key steps of medical device validation. As part of design and development validation, the organization shall perform clinical evaluations or performance. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. What are medical device companies requesting. Medical Device Design Validation.
From www.perforce.com
Design Verification/Validation for Med Device Development Perforce Medical Device Design Validation It’s easy to confuse the two, but there’s a simple way to remember them: What are medical device companies requesting these days when it comes to validation and verification? Medical device validation determines whether you built the right product for its intended use. As part of design and development validation, the organization shall perform clinical evaluations or performance. Design controls. Medical Device Design Validation.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Design Validation Learn the benefits and key steps of medical device validation. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Medical device validation determines whether you built the right product for its intended use. Design verification and validation are two essential steps in medical device product development. As part of design and development validation, the. Medical Device Design Validation.
From www.youtube.com
Developing a Testing Plan for Medical Device Design Verification YouTube Medical Device Design Validation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. As part of design and development validation, the organization shall perform clinical evaluations or performance. Design verification and validation are two. Medical Device Design Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Medical device validation determines whether you built the right product for its intended use. Safe medical device act of 1990 authorized fda to add design controls to the current good. Medical Device Design Validation.
From www.proplate.com
Medical Device Design & Development Electroplating Solutions ProPlate Medical Device Design Validation Learn the benefits and key steps of medical device validation. With this guide, i plan to share valuable insights to explain what design. It’s easy to confuse the two, but there’s a simple way to remember them: Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device act of 1990 authorized fda. Medical Device Design Validation.
From www.emergobyul.com
Analysis of design validation and verification for medical device human Medical Device Design Validation Design verification and validation are two essential steps in medical device product development. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. What are medical device companies requesting these days when it comes to validation and verification? Medical device validation determines whether you built the right product for its intended use. It’s easy to. Medical Device Design Validation.
From arrotek.com
Medical Device Design Validation Explained Arrotek Medical Device Medical Device Design Validation What are medical device companies requesting these days when it comes to validation and verification? Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Learn the benefits and key steps of medical device validation. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use.. Medical Device Design Validation.
From www.greenlight.guru
The Beginner's Guide to Design Verification and Design Validation for Medical Device Design Validation Design verification and validation are two essential steps in medical device product development. As part of design and development validation, the organization shall perform clinical evaluations or performance. Learn the benefits and key steps of medical device validation. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design controls demonstrate. Medical Device Design Validation.
From www.researchandmarkets.com
Understanding FDA Design Verification and Validation Requirements for Medical Device Design Validation Medical device validation determines whether you built the right product for its intended use. With this guide, i plan to share valuable insights to explain what design. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice. Medical Device Design Validation.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Design Validation Learn the benefits and key steps of medical device validation. Design verification and validation are two essential steps in medical device product development. What are medical device companies requesting these days when it comes to validation and verification? If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation. Design controls demonstrate. Medical Device Design Validation.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Validation Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. What are medical device companies requesting these days when it comes to validation and verification? Design verification and validation are two essential steps in medical. Medical Device Design Validation.