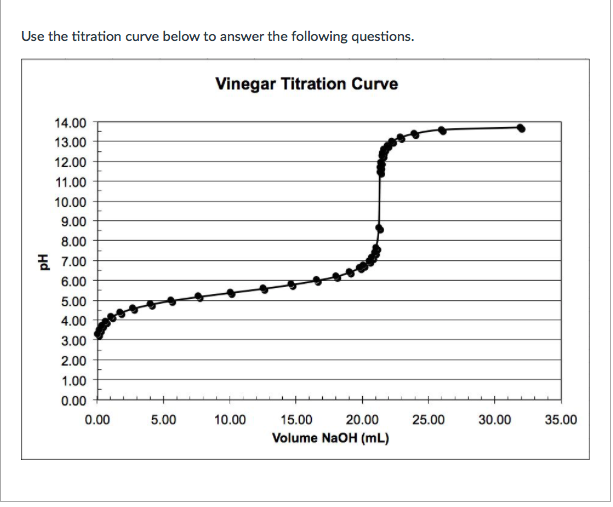
Vinegar Titration Curve . Since we are using a known concentration of base, the technique is also referred to as alkalimetry. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. [h 3o+][a−] = [ha] or. The equilibrium expression for this reaction is: in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. we will use the base solution to titrate the vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. titration curve for the titration of a strong acid with a strong base.
from www.chegg.com
in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. titration curve for the titration of a strong acid with a strong base. The equilibrium expression for this reaction is: Since we are using a known concentration of base, the technique is also referred to as alkalimetry. we will use the base solution to titrate the vinegar solution. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. [h 3o+][a−] = [ha] or. in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely.
Solved Use the titration curve below to answer the following
Vinegar Titration Curve in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. titration curve for the titration of a strong acid with a strong base. we will use the base solution to titrate the vinegar solution. in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. [h 3o+][a−] = [ha] or. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. The equilibrium expression for this reaction is: acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve Since we are using a known concentration of base, the technique is also referred to as alkalimetry. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. titration curve for the titration of a strong acid with a strong base. in this method, you. Vinegar Titration Curve.
From www.chegg.com
Solved Vinegar Titration and Titration Curves In the video I Vinegar Titration Curve you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acid content of vinegar can vary widely, but for table vinegar it typically. Vinegar Titration Curve.
From www.chegg.com
Solved Below is a titration curve for 25.0 mL vinegar with Vinegar Titration Curve titration curve for the titration of a strong acid with a strong base. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve we will use the base solution to titrate the vinegar solution. in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. [h 3o+][a−] = [ha] or. you will measure out a small volume of vinegar and use a. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. titration curve for the titration of a strong acid with a strong base. we will use the. Vinegar Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. titration curve for the titration of a strong acid with a. Vinegar Titration Curve.
From www.sciencebuddies.org
Measuring the Amount of Acid in Vinegar by Titration with an Indicator Vinegar Titration Curve The equilibrium expression for this reaction is: Since we are using a known concentration of base, the technique is also referred to as alkalimetry. titration curve for the titration of a strong acid with a strong base. we will use the base solution to titrate the vinegar solution. in this method, you will manually titrate ch 3. Vinegar Titration Curve.
From dxugqkzdeco.blob.core.windows.net
Titration For Acetic Acid In Vinegar Lab Hol at Kathleen Williamson blog Vinegar Titration Curve [h 3o+][a−] = [ha] or. we will use the base solution to titrate the vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in. Vinegar Titration Curve.
From www.youtube.com
Titration of Vinegar Part 1 YouTube Vinegar Titration Curve in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. titration curve for the titration of a strong acid with a strong base. acid content of vinegar can vary widely, but for table vinegar it typically ranges from. Vinegar Titration Curve.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Vinegar Titration Curve titration curve for the titration of a strong acid with a strong base. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. you will measure out a small volume. Vinegar Titration Curve.
From www.youtube.com
Titration of Vinegar Video Lesson YouTube YouTube Vinegar Titration Curve The equilibrium expression for this reaction is: you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. we. Vinegar Titration Curve.
From www.chegg.com
Experiment 8 TITRATION CURVESVINEGAR TITRATION Vinegar Titration Curve Since we are using a known concentration of base, the technique is also referred to as alkalimetry. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. in this method, you will manually titrate ch 3 co 2 h (aq) with some. Vinegar Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Vinegar Titration Curve in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. The equilibrium expression for this reaction is: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. . Vinegar Titration Curve.
From www.chegg.com
Solved Vinegar Titration and Titration Curves In the video I Vinegar Titration Curve titration curve for the titration of a strong acid with a strong base. [h 3o+][a−] = [ha] or. in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. you will measure out a small volume of vinegar and. Vinegar Titration Curve.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 Vinegar Titration Curve acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. titration curve for the titration of a strong acid with a strong base. we will use the base solution to titrate the vinegar solution. The equilibrium expression for this reaction is: [h 3o+][a−] = [ha] or. . Vinegar Titration Curve.
From www.chegg.com
Experiment 8 TITRATION CURVESVINEGAR TITRATION Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. The equilibrium expression for this reaction is: titration curve for the titration of a strong acid with a strong base.. Vinegar Titration Curve.
From studylib.net
Analysis of Vinegar by Titration Vinegar Titration Curve acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. we will use the base solution to titrate the vinegar solution. in this experiment, a. Vinegar Titration Curve.
From www.chegg.com
Solved 1. Based on the vinegar titration curve, what is the Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. you will measure out a small volume of vinegar and use a burette to determine the. Vinegar Titration Curve.
From www.chegg.com
Solved EXPERIMENT 16A DETERMINATI ACETIC ACID IN VINEGAR BY Vinegar Titration Curve Since we are using a known concentration of base, the technique is also referred to as alkalimetry. we will use the base solution to titrate the vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. titration curve for. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve in this method, you will manually titrate ch 3 co 2 h (aq) with some amount of naoh (aq) to find out the concentration of the given vinegar solution. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. acid content of vinegar can vary widely, but for table vinegar it. Vinegar Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. Since we are using a known concentration of base, the technique. Vinegar Titration Curve.
From article.sapub.org
On the Titration of a Weak Acid with a Weak Base Application to the Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. [h 3o+][a−] = [ha] or. in this experiment, a technique known as a titration will be. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation ID2417858 Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. titration curve for the titration of a strong acid with a strong base. we will use the base solution to titrate the vinegar solution. The equilibrium expression for this reaction is: in this experiment, a. Vinegar Titration Curve.
From www.coursehero.com
[Solved] You have performed a titration of vinegar using a burette and Vinegar Titration Curve we will use the base solution to titrate the vinegar solution. The equilibrium expression for this reaction is: [h 3o+][a−] = [ha] or. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. Since we are using a known concentration of base, the technique is. Vinegar Titration Curve.
From www.chegg.com
Solved Vinegar Titration and Titration Curves In the video I Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The equilibrium expression for this reaction is: titration curve for the titration of a strong acid with a strong base. in this method, you will manually titrate ch 3 co 2 h (aq) with some amount. Vinegar Titration Curve.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Vinegar Titration Curve acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The equilibrium expression for this reaction is: [h 3o+][a−] = [ha] or. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this method, you. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve The equilibrium expression for this reaction is: we will use the base solution to titrate the vinegar solution. titration curve for the titration of a strong acid with a strong base. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in this experiment, a technique. Vinegar Titration Curve.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Vinegar Titration Curve titration curve for the titration of a strong acid with a strong base. The equilibrium expression for this reaction is: we will use the base solution to titrate the vinegar solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acid content of vinegar. Vinegar Titration Curve.
From www.chegg.com
Solved Use the titration curve below to answer the following Vinegar Titration Curve [h 3o+][a−] = [ha] or. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. titration curve for the titration of a strong acid with a strong base. The equilibrium expression for this reaction is: in this method, you will manually titrate ch 3 co 2. Vinegar Titration Curve.
From www.chegg.com
Solved A student generated the titration curve shown below. Vinegar Titration Curve The equilibrium expression for this reaction is: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. in this experiment, a technique known. Vinegar Titration Curve.
From www.numerade.com
SOLVED Acetic Acid in Vinegar Titration Data mL of vinegar added to 45 Vinegar Titration Curve Since we are using a known concentration of base, the technique is also referred to as alkalimetry. you will measure out a small volume of vinegar and use a burette to determine the volume of sodium hydroxide required to completely. in this experiment, a technique known as a titration will be used to determine the concentration of acetic. Vinegar Titration Curve.
From www.chegg.com
Solved Vinegar titration curve 5 1.5 points Use the Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Since we are using a known concentration of base, the technique is also referred to as. Vinegar Titration Curve.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Vinegar Titration Curve in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. [h 3o+][a−] = [ha] or. Since we are using a known concentration of base, the technique is also referred to as alkalimetry. we will use the base solution to titrate the vinegar solution. acid content of. Vinegar Titration Curve.
From www.slideserve.com
PPT Strength of Vinegar by AcidBase Titration PowerPoint Vinegar Titration Curve Since we are using a known concentration of base, the technique is also referred to as alkalimetry. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The equilibrium expression for this reaction is: acid content of vinegar can vary widely, but for table vinegar it typically. Vinegar Titration Curve.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5570905 Vinegar Titration Curve titration curve for the titration of a strong acid with a strong base. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Since we are. Vinegar Titration Curve.