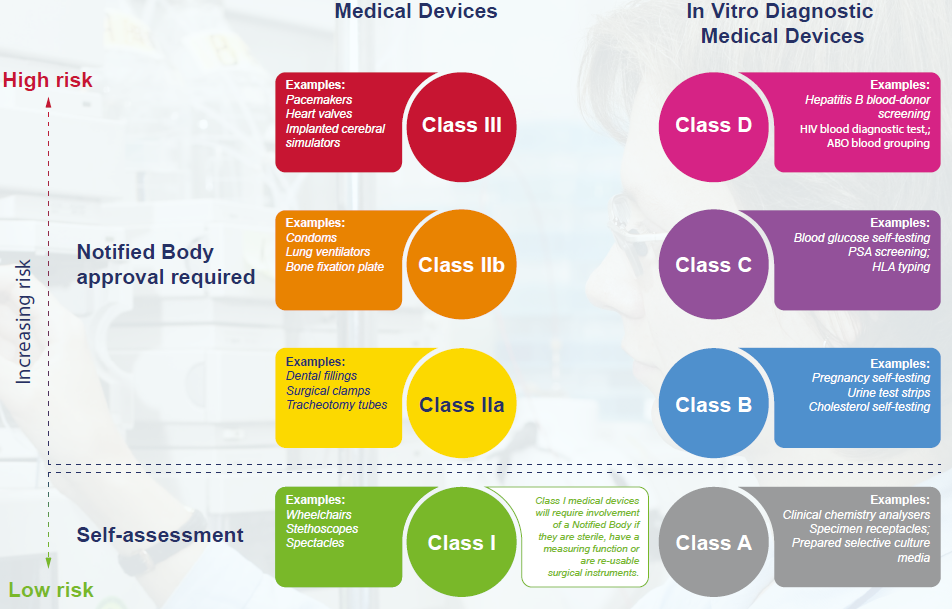
Device Classification Regulations . Of standard for medical devices office of standards and guidelines development. Medical device regulations and utilization of international standards in japan. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based on the risk level; Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. Medical device classification and regulation.
from www.biosliceblog.com
Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. Medical device classification and regulation. Of standard for medical devices office of standards and guidelines development. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and.
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog
Device Classification Regulations The food and drug administration (fda) has established. In japan, medical devices are classified into four classes based on the risk level; Medical device regulations and utilization of international standards in japan. Medical device classification and regulation. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. The food and drug administration (fda) has established. Of standard for medical devices office of standards and guidelines development. Mds are classified into 4 categories (class i to iv) according to risk level.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Device Classification Regulations The food and drug administration (fda) has established. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based on the risk level; Mds are classified into 4 categories (class i to iv) according to risk level. Medical device regulations and. Device Classification Regulations.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Device Classification Regulations A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. Of standard for medical devices office of standards and guidelines development. Medical device classification and regulation. Mds are. Device Classification Regulations.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. Of standard for medical devices office of standards and guidelines development. Medical device classification and regulation. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based. Device Classification Regulations.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Classification Regulations A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Of standard for medical devices office of standards and guidelines development. Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are. Device Classification Regulations.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Device Classification Regulations The food and drug administration (fda) has established. Mds are classified into 4 categories (class i to iv) according to risk level. Medical device regulations and utilization of international standards in japan. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and. Device Classification Regulations.
From www.greenlight.guru
Ultimate Guide to Software as a Medical Device (SaMD) Device Classification Regulations In japan, medical devices are classified into four classes based on the risk level; Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories (class i to iv) according to risk level. Of standard for medical devices office of standards and guidelines development. The food and drug administration (fda) has established. Medical device classification. Device Classification Regulations.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and guidelines development. A device is in class ii if general controls alone are insufficient to provide reasonable assurance. Device Classification Regulations.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Device Classification Regulations Medical device classification and regulation. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. Mds are classified into 4 categories (class i to iv) according to risk. Device Classification Regulations.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. Of standard for medical devices office of standards and guidelines development. Medical device classification and regulation. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. In japan, medical devices are classified into four classes based. Device Classification Regulations.
From innolitics.com
2013 FDA Guidance Medical Device Classification Product Codes Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. Medical device regulations and utilization of international standards in japan. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. A device is in class ii if general controls. Device Classification Regulations.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Device Classification Regulations A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. The food and drug administration (fda) has established. Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical. Device Classification Regulations.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Device Classification Regulations A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical device regulations and utilization of international standards in japan. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. Medical device classification and regulation. Mds are classified. Device Classification Regulations.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Device Classification Regulations Medical device classification and regulation. Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. Medical device regulations and utilization of international standards in japan. Of standard for medical devices office of standards and guidelines development. A device is in class ii if general controls alone are insufficient. Device Classification Regulations.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Device Classification Regulations Medical device regulations and utilization of international standards in japan. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and guidelines development. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and.. Device Classification Regulations.
From medicaldevicehq.com
Different classifications rules for medical device software An Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. The food and drug administration (fda) has established. Medical device regulations and. Device Classification Regulations.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Classification Regulations Of standard for medical devices office of standards and guidelines development. In japan, medical devices are classified into four classes based on the risk level; Mds are classified into 4 categories (class i to iv) according to risk level. Medical device regulations and utilization of international standards in japan. The food and drug administration (fda) has established. Medical device classification. Device Classification Regulations.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and guidelines development. Medical device regulations and utilization of international standards in japan. Medical device classification. Device Classification Regulations.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Device Classification Regulations Mds are classified into 4 categories (class i to iv) according to risk level. Of standard for medical devices office of standards and guidelines development. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. A device is in class ii if general. Device Classification Regulations.
From ciqa.net
How to Determine the Medical Device Classification? • Follow us for more. Device Classification Regulations A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. The food and drug administration (fda) has established. Medical device regulations and utilization of international standards in japan. Medical device classification and regulation. Of standard for medical devices office of standards and guidelines development. Mds are classified into 4 categories. Device Classification Regulations.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Device Classification Regulations Of standard for medical devices office of standards and guidelines development. Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. A device is in class ii if general controls alone are insufficient to. Device Classification Regulations.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Classification Regulations Of standard for medical devices office of standards and guidelines development. Medical device regulations and utilization of international standards in japan. The food and drug administration (fda) has established. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of. Device Classification Regulations.
From www.researchgate.net
Medical Device Classification System Download Table Device Classification Regulations The food and drug administration (fda) has established. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical device regulations and utilization of international standards in japan. Of standard for. Device Classification Regulations.
From english.nmpa.gov.cn
Rules for Classification of Medical Devices Device Classification Regulations Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. The food. Device Classification Regulations.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Device Classification Regulations Of standard for medical devices office of standards and guidelines development. Mds are classified into 4 categories (class i to iv) according to risk level. Medical device regulations and utilization of international standards in japan. The food and drug administration (fda) has established. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of. Device Classification Regulations.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Device Classification Regulations Of standard for medical devices office of standards and guidelines development. In japan, medical devices are classified into four classes based on the risk level; Mds are classified into 4 categories (class i to iv) according to risk level. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical. Device Classification Regulations.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Device Classification Regulations The food and drug administration (fda) has established. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical device classification and regulation. Mds are classified into 4 categories (class i to iv) according to risk. Device Classification Regulations.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Device Classification Regulations The food and drug administration (fda) has established. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Of standard for medical devices office of standards and guidelines development. Medical device classification and regulation. Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories. Device Classification Regulations.
From www.presentationeze.com
TGA Medical Device Classification Rule 5 Special RulesPresentationEZE Device Classification Regulations The food and drug administration (fda) has established. Medical device regulations and utilization of international standards in japan. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Of standard for medical devices office of standards and guidelines development. In japan, medical devices are classified into four classes based on. Device Classification Regulations.
From familyclinic.netlify.app
Medical device regulations fda Device Classification Regulations The food and drug administration (fda) has established. Mds are classified into 4 categories (class i to iv) according to risk level. Medical device classification and regulation. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and guidelines development. A device is in class ii if general. Device Classification Regulations.
From www.castoredc.com
Overview of EU Medical Device Regulations (MDR) Device Classification Regulations The food and drug administration (fda) has established. Medical device regulations and utilization of international standards in japan. In japan, medical devices are classified into four classes based on the risk level; Of standard for medical devices office of standards and guidelines development. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of. Device Classification Regulations.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Device Classification Regulations The food and drug administration (fda) has established. In japan, medical devices are classified into four classes based on the risk level; A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical device regulations and utilization of international standards in japan. Of standard for medical devices office of standards. Device Classification Regulations.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Device Classification Regulations Medical device classification and regulation. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. Medical device regulations and utilization of international standards in japan. Of standard for. Device Classification Regulations.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Device Classification Regulations The food and drug administration (fda) has established. Of standard for medical devices office of standards and guidelines development. Medical device regulations and utilization of international standards in japan. A device is in class ii if general controls alone are insufficient to provide reasonable assurance of its safety and. Medical device classification and regulation. In japan, medical devices are classified. Device Classification Regulations.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Device Classification Regulations Medical device classification and regulation. Mds are classified into 4 categories (class i to iv) according to risk level. In japan, medical devices are classified into four classes based on the risk level; The food and drug administration (fda) has established. Of standard for medical devices office of standards and guidelines development. A device is in class ii if general. Device Classification Regulations.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Device Classification Regulations In japan, medical devices are classified into four classes based on the risk level; Medical device regulations and utilization of international standards in japan. Mds are classified into 4 categories (class i to iv) according to risk level. The food and drug administration (fda) has established. Of standard for medical devices office of standards and guidelines development. Medical device classification. Device Classification Regulations.