What Is A Triple Covalent Bond . Draw lewis structures of covalent molecules that contain double and triple bonds. A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. Explain how double and triple bonds are formed in covalent molecules. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. A triple covalent bond is when three pairs of electrons, or six electrons,.
from askfilo.com
The covalent bond between two nitrogen atom involves. A triple covalent bond is when three pairs of electrons, or six electrons,. A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. Draw lewis structures of covalent molecules that contain double and triple bonds.
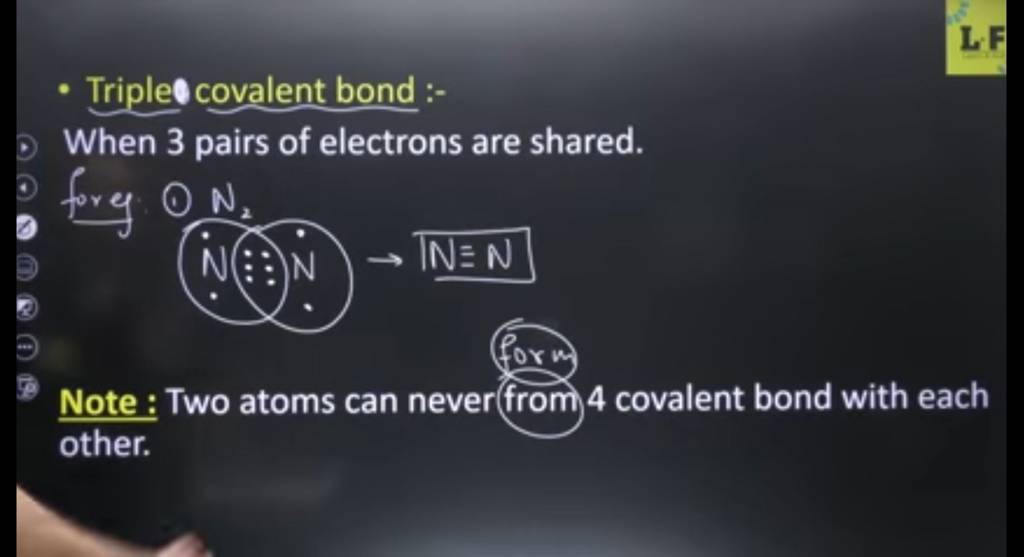
Triple covalent bond When 3 pairs of electrons are shared. forey (1)..
What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. A triple covalent bond is when three pairs of electrons, or six electrons,. The covalent bond between two nitrogen atom involves. Draw lewis structures of covalent molecules that contain double and triple bonds.
From sciencenotes.org
Single, Double, and Triple Bonds What Is A Triple Covalent Bond A triple covalent bond is when three pairs of electrons, or six electrons,. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. A covalent. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID4003633 What Is A Triple Covalent Bond Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in. What Is A Triple Covalent Bond.
From mavink.com
Covalent Bond Types What Is A Triple Covalent Bond Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A triple covalent bond is when three pairs of electrons, or six electrons,. Explain how double and triple bonds are formed in covalent molecules. A covalent bond is a type of chemical bond that involves the sharing. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID5619918 What Is A Triple Covalent Bond Explain how double and triple bonds are formed in covalent molecules. A triple covalent bond is when three pairs of electrons, or six electrons,. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. A covalent bond is a type of chemical bond that involves the sharing of. What Is A Triple Covalent Bond.
From www.chemistrylearner.com
Triple Covalent Bond Definition and Examples What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. Explain how double and triple bonds are formed in covalent molecules. A covalent bond is a type of chemical bond that involves the sharing of electron. What Is A Triple Covalent Bond.
From www.youtube.com
What is meant by triple covalent bond? science chemistry triple What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A covalent bond is a type of chemical bond that involves the sharing of electron. What Is A Triple Covalent Bond.
From askfilo.com
Triple covalent bond When 3 pairs of electrons are shared. forey (1).. What Is A Triple Covalent Bond A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. A triple covalent bond is when three pairs of electrons, or six electrons,. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms. What Is A Triple Covalent Bond.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Is A Triple Covalent Bond Draw lewis structures of covalent molecules that contain double and triple bonds. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. The covalent bond between two nitrogen atom involves. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom.. What Is A Triple Covalent Bond.
From www.adda247.com
Covalent Bond Definition, Examples for Class 10 What Is A Triple Covalent Bond A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Triple covalent bond is the bond that is. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation What Is A Triple Covalent Bond Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. The covalent bond between two nitrogen atom involves. A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two. What Is A Triple Covalent Bond.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds What Is A Triple Covalent Bond The covalent bond between two nitrogen atom involves. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. A triple covalent bond is when atoms share three pairs of electrons. Draw lewis structures of covalent molecules that contain double and triple bonds. Explain how double and triple bonds are formed in covalent. What Is A Triple Covalent Bond.
From www.expii.com
Multiple Bonds — Double & Triple Bonds Expii What Is A Triple Covalent Bond The covalent bond between two nitrogen atom involves. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Explain how double and triple bonds are formed in covalent molecules.. What Is A Triple Covalent Bond.
From chemizi.blogspot.com
Covalent bonddefinitionexamplesformation energy and types What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Draw lewis structures of covalent molecules that contain double and triple bonds. The covalent bond between two nitrogen atom involves.. What Is A Triple Covalent Bond.
From slideplayer.com
Covalent Bonding. ppt download What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. A triple covalent bond is when three pairs of electrons, or six electrons,. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as. What Is A Triple Covalent Bond.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica What Is A Triple Covalent Bond A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. The covalent bond between two nitrogen atom involves. Draw lewis structures of covalent molecules that contain double and triple bonds. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2,. What Is A Triple Covalent Bond.
From www.politicalfunda.com
Covalent Bond Covalent Bond Definition, Types, Properties & Facts What Is A Triple Covalent Bond Draw lewis structures of covalent molecules that contain double and triple bonds. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A triple covalent bond is when three. What Is A Triple Covalent Bond.
From www.pinterest.com
Triple Covalent Bond Easy Science Covalent bonding, Chemistry What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. A triple covalent bond is when three pairs of electrons, or six electrons,. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Draw lewis structures of covalent molecules that. What Is A Triple Covalent Bond.
From chemistryrack.com
What is a triple covalent bond? ChemistryRack What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. A triple covalent bond is when atoms share three pairs of electrons. A triple covalent bond is when three pairs of electrons, or six electrons,. Explain how double and triple bonds are formed in covalent molecules. The covalent. What Is A Triple Covalent Bond.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy What Is A Triple Covalent Bond A triple covalent bond is when three pairs of electrons, or six electrons,. A triple covalent bond is when atoms share three pairs of electrons. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Triple covalent bond is the bond that is formed by sharing of. What Is A Triple Covalent Bond.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Draw lewis structures of covalent molecules that. What Is A Triple Covalent Bond.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Covalent Bond The covalent bond between two nitrogen atom involves. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Draw lewis structures of covalent molecules that contain double and triple bonds. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID2192070 What Is A Triple Covalent Bond Explain how double and triple bonds are formed in covalent molecules. Draw lewis structures of covalent molecules that contain double and triple bonds. A triple covalent bond is when three pairs of electrons, or six electrons,. A triple covalent bond is when atoms share three pairs of electrons. Triple bond, in chemistry, a covalent linkage in which two atoms share. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Draw lewis structures of covalent molecules that contain double and triple bonds.. What Is A Triple Covalent Bond.
From www.dreamstime.com
Three Types of Covalent Bonds Including Single, Double, and Triple What Is A Triple Covalent Bond Draw lewis structures of covalent molecules that contain double and triple bonds. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. A triple covalent bond is when atoms share three pairs of electrons. Triple covalent bond is the bond that. What Is A Triple Covalent Bond.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Covalent Bond The covalent bond between two nitrogen atom involves. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Draw lewis structures of covalent molecules that contain double and triple bonds. Explain how double and triple bonds are formed in covalent molecules. A triple covalent bond is when. What Is A Triple Covalent Bond.
From biologydictionary.net
Covalent Bond Biology Dictionary What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. A triple covalent bond is when three pairs of electrons, or six electrons,. Explain how double and triple bonds are formed in covalent molecules. Triple. What Is A Triple Covalent Bond.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. Explain how double and triple bonds are formed in covalent molecules. A. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236 What Is A Triple Covalent Bond Explain how double and triple bonds are formed in covalent molecules. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. A triple covalent bond is when atoms share three pairs of electrons. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the. What Is A Triple Covalent Bond.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Covalent Bond Draw lewis structures of covalent molecules that contain double and triple bonds. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. A triple covalent bond is when atoms share three pairs of electrons. A triple covalent bond is when three pairs of electrons, or six electrons,. Triple covalent bond is the. What Is A Triple Covalent Bond.
From www.youtube.com
Formation of Triple Covalent Bonds YouTube What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. A triple covalent bond is when three pairs of electrons, or six electrons,. The covalent bond between two nitrogen atom involves. Draw lewis structures of covalent molecules that contain double and triple bonds. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of. What Is A Triple Covalent Bond.
From www.youtube.com
carbon and its compounds class 10 triple covalent bond YouTube What Is A Triple Covalent Bond Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. The covalent bond between two nitrogen atom involves. Explain how double and triple bonds are formed in covalent molecules. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms. What Is A Triple Covalent Bond.
From www.slideserve.com
PPT Covalent Bonding & Molecular Compounds PowerPoint Presentation What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. Draw lewis structures of covalent molecules that contain double and triple bonds. A triple covalent bond is when three pairs of electrons, or six electrons,. A triple covalent bond is. What Is A Triple Covalent Bond.
From slideplayer.com
Single, Double, and Triple Bonds Covalent Bonds. ppt download What Is A Triple Covalent Bond Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. Triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, n2, or. Explain how double and triple bonds are. What Is A Triple Covalent Bond.
From www.youtube.com
What is Triple Covalent Bond Class 10 Chemistry chemistry What Is A Triple Covalent Bond A triple covalent bond is when atoms share three pairs of electrons. Explain how double and triple bonds are formed in covalent molecules. Triple covalent bond is the bond that is formed by sharing of three electron pairs between two atoms in which each atom. The covalent bond between two nitrogen atom involves. Draw lewis structures of covalent molecules that. What Is A Triple Covalent Bond.
From www.youtube.com
Electron dot structure of N2 molecule Triple bond formation in N2 What Is A Triple Covalent Bond Explain how double and triple bonds are formed in covalent molecules. Draw lewis structures of covalent molecules that contain double and triple bonds. A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. The covalent bond between two nitrogen atom involves. A triple covalent bond is when three pairs of electrons, or. What Is A Triple Covalent Bond.