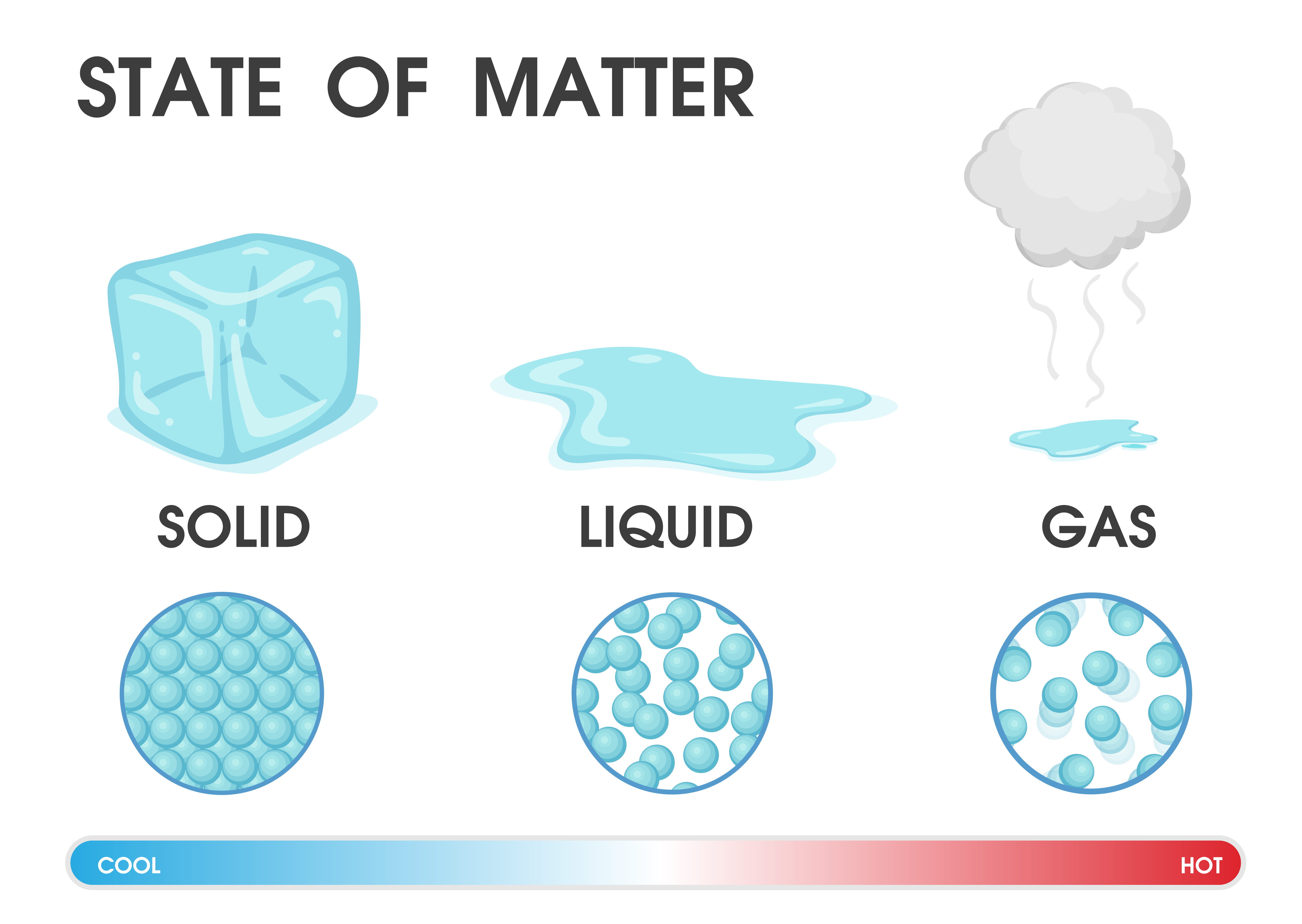
Which Liquid Changes Into Solid On Heating . Chocolate melts at around 35°c. The heat added at the melting point is used to change the To calculate the energy changes that accompany phase changes. A liquid can freeze into a solid or vaporize into a gas. Liquids do not have to be heated to. Plasma can deionize or recombine to. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Melting, freezing, evaporating and condensing. As the ice heats up, it melts and turns into water. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. There are four main changes of state: Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. A gas can deposit into a solid, condense into a liquid, or ionize into plasma.
from www.vecteezy.com
Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. Melting, freezing, evaporating and condensing. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. The heat added at the melting point is used to change the Liquids do not have to be heated to. There are four main changes of state: A gas can deposit into a solid, condense into a liquid, or ionize into plasma. A liquid can freeze into a solid or vaporize into a gas. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Chocolate melts at around 35°c.
Changing the state of matter from solid, liquid and gas due to
Which Liquid Changes Into Solid On Heating Chocolate melts at around 35°c. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Melting, freezing, evaporating and condensing. As the ice heats up, it melts and turns into water. There are four main changes of state: Plasma can deionize or recombine to. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Chocolate melts at around 35°c. The heat added at the melting point is used to change the A liquid can freeze into a solid or vaporize into a gas. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. To calculate the energy changes that accompany phase changes. Liquids do not have to be heated to. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. The heat added at the melting point is used to change the Liquids do not have to be. Which Liquid Changes Into Solid On Heating.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Which Liquid Changes Into Solid On Heating A liquid can freeze into a solid or vaporize into a gas. Plasma can deionize or recombine to. To calculate the energy changes that accompany phase changes. There are four main changes of state: Chocolate melts at around 35°c. The heat added at the melting point is used to change the A gas can deposit into a solid, condense into. Which Liquid Changes Into Solid On Heating.
From www.scribd.com
The Effect of Heat On Matter PDF Liquids Solid Which Liquid Changes Into Solid On Heating When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Plasma can deionize or recombine to. There are four main changes of state: The heat added at the melting point is used to change the Specific latent heat is the amount of energy. Which Liquid Changes Into Solid On Heating.
From www.snexplores.org
Explainer What are the different states of matter? Which Liquid Changes Into Solid On Heating A liquid can freeze into a solid or vaporize into a gas. To calculate the energy changes that accompany phase changes. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Melting, freezing, evaporating and condensing. Chocolate melts at around 35°c. Specific latent heat is the amount of. Which Liquid Changes Into Solid On Heating.
From www.britannica.com
phase Definition & Facts Britannica Which Liquid Changes Into Solid On Heating To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. Chocolate melts at around 35°c. Melting, freezing, evaporating. Which Liquid Changes Into Solid On Heating.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Specific latent heat is the amount of energy required to change the state. Which Liquid Changes Into Solid On Heating.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Which Liquid Changes Into Solid On Heating When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Plasma can deionize or recombine to. To calculate the energy changes that accompany phase changes. Chocolate melts at around 35°c. Melting is when a solid changes into a liquid requires heat energy which. Which Liquid Changes Into Solid On Heating.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Which Liquid Changes Into Solid On Heating To calculate the energy changes that accompany phase changes. There are four main changes of state: As the ice heats up, it melts and turns into water. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Chocolate melts at around 35°c. A liquid can freeze into a. Which Liquid Changes Into Solid On Heating.
From classnotes123.com
Solid Liquid and Gases Class 5 CBSE Class Notes Online Classnotes123 Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. As the ice heats up, it melts and turns into water. Chocolate melts at around 35°c. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. A liquid. Which Liquid Changes Into Solid On Heating.
From www.edplace.com
Describe Processes of Heat Transfer Worksheet EdPlace Which Liquid Changes Into Solid On Heating When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. A liquid can freeze into a solid or vaporize into a gas. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to. Which Liquid Changes Into Solid On Heating.
From www.youtube.com
To show that solids, liquids, and gases expand on heating YouTube Which Liquid Changes Into Solid On Heating A liquid can freeze into a solid or vaporize into a gas. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Plasma can deionize or recombine to.. Which Liquid Changes Into Solid On Heating.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID1331141 Which Liquid Changes Into Solid On Heating Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. There are four main changes of state: A liquid can freeze into a solid or vaporize into a gas. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further,. Which Liquid Changes Into Solid On Heating.
From mungfali.com
Solid Liquid Gas Anchor Chart Which Liquid Changes Into Solid On Heating A liquid can freeze into a solid or vaporize into a gas. The heat added at the melting point is used to change the There are four main changes of state: Melting, freezing, evaporating and condensing. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to. Which Liquid Changes Into Solid On Heating.
From preparatorychemistry.com
Heating Curve Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. The heat added at the melting point is used to change the When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Chocolate. Which Liquid Changes Into Solid On Heating.
From www.doubtnut.com
Liquid changes into solid ,On heating to its boiling point Which Liquid Changes Into Solid On Heating The heat added at the melting point is used to change the Plasma can deionize or recombine to. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Chocolate melts at around 35°c. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes. Which Liquid Changes Into Solid On Heating.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Which Liquid Changes Into Solid On Heating A liquid can freeze into a solid or vaporize into a gas. Melting, freezing, evaporating and condensing. Plasma can deionize or recombine to. Liquids do not have to be heated to. As the ice heats up, it melts and turns into water. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice. Which Liquid Changes Into Solid On Heating.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Which Liquid Changes Into Solid On Heating Melting, freezing, evaporating and condensing. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Liquids do not have to be heated to. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. As the. Which Liquid Changes Into Solid On Heating.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. The heat added at the melting point is used to change the To calculate the energy changes that accompany phase changes. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but. Which Liquid Changes Into Solid On Heating.
From www.vecteezy.com
Three different States of matter solid, liquid and gasuas state. Inter Which Liquid Changes Into Solid On Heating Liquids do not have to be heated to. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. There are. Which Liquid Changes Into Solid On Heating.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Which Liquid Changes Into Solid On Heating Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. Chocolate melts at around 35°c. Plasma can deionize or recombine to. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. To calculate the energy changes that. Which Liquid Changes Into Solid On Heating.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Which Liquid Changes Into Solid On Heating Liquids do not have to be heated to. As the ice heats up, it melts and turns into water. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. To calculate the energy changes that accompany phase changes. Plasma can deionize or recombine to. Melting is when. Which Liquid Changes Into Solid On Heating.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Which Liquid Changes Into Solid On Heating Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Liquids do not have to be heated to. When the temperature reaches the melting point of the solid upon heating, the temperature does. Which Liquid Changes Into Solid On Heating.
From ch301.cm.utexas.edu
heating curve Which Liquid Changes Into Solid On Heating Melting, freezing, evaporating and condensing. There are four main changes of state: Chocolate melts at around 35°c. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. Liquids do not have to be heated to. Specific latent heat is the amount of energy. Which Liquid Changes Into Solid On Heating.
From slideplayer.com
Changes in states of matter pt.1 ppt download Which Liquid Changes Into Solid On Heating Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. Liquids do not have to be heated to. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. As the ice heats up, it melts and turns. Which Liquid Changes Into Solid On Heating.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Which Liquid Changes Into Solid On Heating Liquids do not have to be heated to. There are four main changes of state: We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. A liquid can freeze into a solid or vaporize into a gas. Melting, freezing, evaporating and condensing. A gas can deposit into a solid, condense. Which Liquid Changes Into Solid On Heating.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Which Liquid Changes Into Solid On Heating Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. To calculate the energy changes that accompany phase changes. A liquid can freeze into a solid or vaporize into a gas. Specific latent heat is the amount of energy required to change the state of 1 kg of. Which Liquid Changes Into Solid On Heating.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind Which Liquid Changes Into Solid On Heating Plasma can deionize or recombine to. Melting, freezing, evaporating and condensing. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. We take advantage of changes between the gas, liquid, and solid states. Which Liquid Changes Into Solid On Heating.
From easyscienceforkids.com
Changes in Matter Phases States of Matter Image Which Liquid Changes Into Solid On Heating Liquids do not have to be heated to. As the ice heats up, it melts and turns into water. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. Chocolate melts at. Which Liquid Changes Into Solid On Heating.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Which Liquid Changes Into Solid On Heating Chocolate melts at around 35°c. Melting, freezing, evaporating and condensing. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Specific latent heat is the amount of energy. Which Liquid Changes Into Solid On Heating.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Which Liquid Changes Into Solid On Heating When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. As the ice heats up, it melts and turns into water. The heat added at the melting point is used to change the Melting, freezing, evaporating and condensing. To calculate the energy changes. Which Liquid Changes Into Solid On Heating.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Which Liquid Changes Into Solid On Heating There are four main changes of state: Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. Chocolate melts at around 35°c. The heat added at the melting point is used to change the To calculate the energy changes that accompany phase changes. Liquids do not have to. Which Liquid Changes Into Solid On Heating.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts Which Liquid Changes Into Solid On Heating Specific latent heat is the amount of energy required to change the state of 1 kg of a material without changing its temperature. As the ice heats up, it melts and turns into water. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. When the temperature reaches the melting point of the solid upon. Which Liquid Changes Into Solid On Heating.
From www.alamy.com
States of matter diagram Stock Vector Images Alamy Which Liquid Changes Into Solid On Heating Plasma can deionize or recombine to. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. The heat added at the melting point is used to change the Melting, freezing, evaporating and condensing. Melting is when a solid changes into a liquid requires. Which Liquid Changes Into Solid On Heating.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry Which Liquid Changes Into Solid On Heating We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Melting is when a solid changes into a liquid requires heat energy which transforms into kinetic energy, allowing the particles to move. When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further,. Which Liquid Changes Into Solid On Heating.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Which Liquid Changes Into Solid On Heating When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. As the ice heats up, it melts and turns into water. Plasma can deionize or recombine to. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. We. Which Liquid Changes Into Solid On Heating.