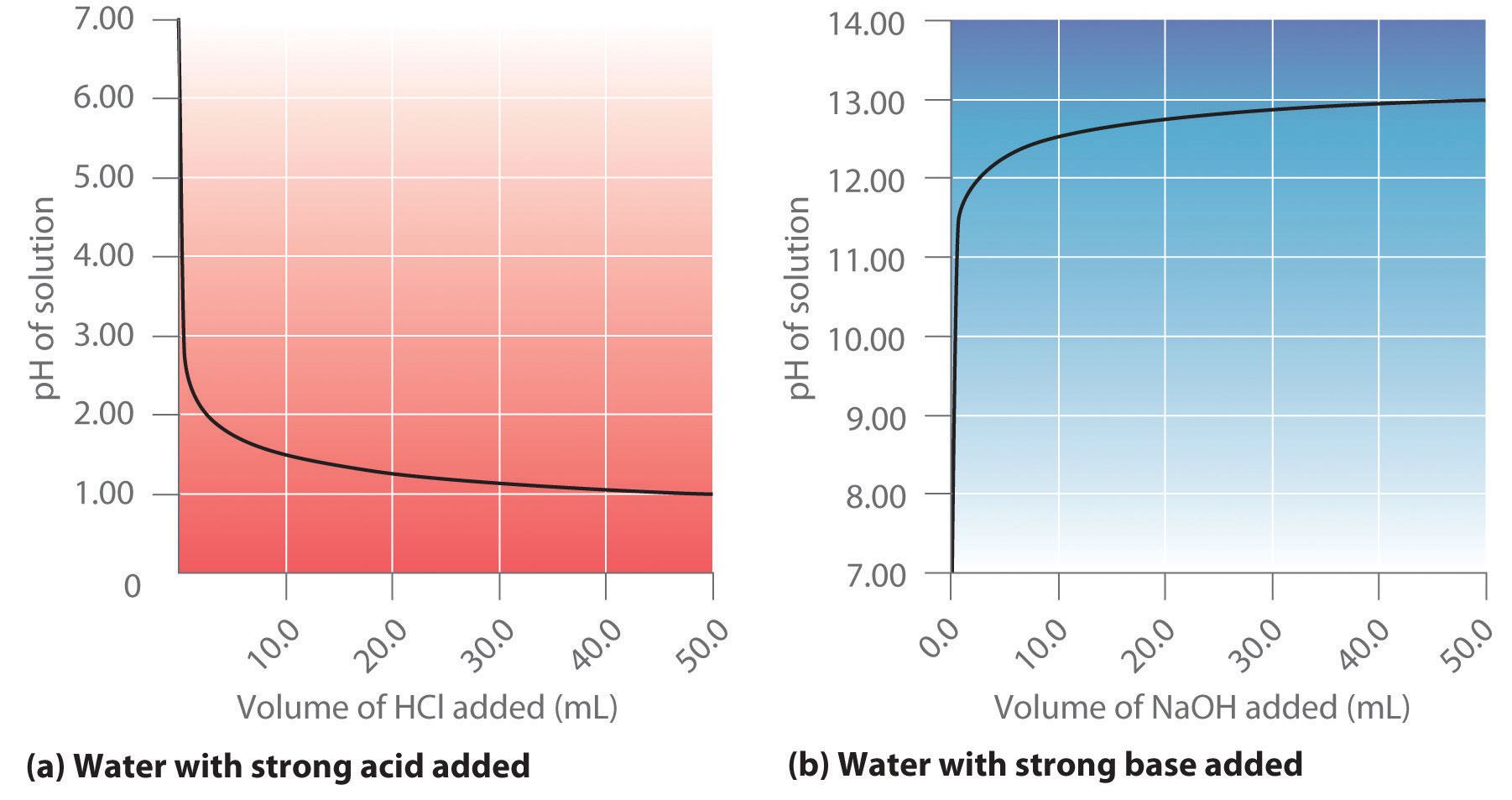
Ph Meter Titration Of Naoh And Hcl . you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? to identify the equivalence point in the titration, we use titration curves and indicators. Determine the equivalence point by. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Use of volumetric flask, and graduated pipet. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. According to the concentration of acid and base solutions, we have.
from saylordotorg.github.io
Use of volumetric flask, and graduated pipet. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. According to the concentration of acid and base solutions, we have. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. to identify the equivalence point in the titration, we use titration curves and indicators. Determine the equivalence point by.
AcidBase Titrations
Ph Meter Titration Of Naoh And Hcl to identify the equivalence point in the titration, we use titration curves and indicators. Determine the equivalence point by. to identify the equivalence point in the titration, we use titration curves and indicators. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Use of volumetric flask, and graduated pipet. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration.
From fphoto.photoshelter.com
science chemistry titration hydrochloric acid sodium hydroxide Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Determine the equivalence point by. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml. Ph Meter Titration Of Naoh And Hcl.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Ph Meter Titration Of Naoh And Hcl Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Determine the equivalence point by. to identify the equivalence point in the titration, we use titration curves and indicators. Use of volumetric flask, and graduated pipet. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been. Ph Meter Titration Of Naoh And Hcl.
From www.researchgate.net
(PDF) Infrared titration of aqueous NaOH by aqueous HCl Ph Meter Titration Of Naoh And Hcl Use of volumetric flask, and graduated pipet. Determine the equivalence point by. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. to identify the equivalence point in the titration, we use titration curves. Ph Meter Titration Of Naoh And Hcl.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Ph Meter Titration Of Naoh And Hcl Determine the equivalence point by. According to the concentration of acid and base solutions, we have. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Titration of 25.0. Ph Meter Titration Of Naoh And Hcl.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Ph Meter Titration Of Naoh And Hcl Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? to identify the equivalence point in the titration, we use titration curves and indicators. Use of volumetric flask, and graduated pipet. you will titrate a solution of hcl with a standardized solution of. Ph Meter Titration Of Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Ph Meter Titration Of Naoh And Hcl to identify the equivalence point in the titration, we use titration curves and indicators. According to the concentration of acid and base solutions, we have. Use of volumetric flask, and graduated pipet. Determine the equivalence point by. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100. Ph Meter Titration Of Naoh And Hcl.
From www.sarthaks.com
which one of the following curves represents the graph of pH during Ph Meter Titration Of Naoh And Hcl Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Determine the equivalence point by. According to the concentration of acid and base solutions, we have. Titration of. Ph Meter Titration Of Naoh And Hcl.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Ph Meter Titration Of Naoh And Hcl to identify the equivalence point in the titration, we use titration curves and indicators. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. According to the concentration of acid and base solutions, we have. Use of volumetric flask, and graduated pipet. Determine the equivalence point by. you will titrate a. Ph Meter Titration Of Naoh And Hcl.
From saylordotorg.github.io
AcidBase Titrations Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\) what is the ph when 48.00. Ph Meter Titration Of Naoh And Hcl.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Ph Meter Titration Of Naoh And Hcl Use of volumetric flask, and graduated pipet. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Determine the equivalence point by. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? to identify the equivalence point. Ph Meter Titration Of Naoh And Hcl.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. Determine the equivalence point by. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? to identify the equivalence point in the titration, we use titration curves and indicators. Use of volumetric flask,. Ph Meter Titration Of Naoh And Hcl.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. to identify the equivalence point in the titration, we use titration curves and indicators. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Blocked areas on the curve indicate the ph range. Ph Meter Titration Of Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Ph Meter Titration Of Naoh And Hcl Determine the equivalence point by. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Use of volumetric flask, and graduated pipet. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\). Ph Meter Titration Of Naoh And Hcl.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Use of volumetric flask, and graduated pipet. According to the concentration of acid and base solutions, we have. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Example \(\pageindex{3}\) what is the ph when. Ph Meter Titration Of Naoh And Hcl.
From www.youtube.com
PH metric titrations of HCl verses NaOH YouTube Ph Meter Titration Of Naoh And Hcl Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. to identify the equivalence point in. Ph Meter Titration Of Naoh And Hcl.
From byjus.com
The graph of pH during the titration of NaOH and HCl Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Determine the equivalence point by. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? you will titrate a solution of hcl. Ph Meter Titration Of Naoh And Hcl.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Ph Meter Titration Of Naoh And Hcl Use of volumetric flask, and graduated pipet. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Determine the equivalence point by. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh. Ph Meter Titration Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Ph Meter Titration Of Naoh And Hcl Determine the equivalence point by. Use of volumetric flask, and graduated pipet. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. to identify the equivalence point in the titration, we use titration curves and indicators. Titration of 25.0 ml of 0.1m hcl by 0.1. Ph Meter Titration Of Naoh And Hcl.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Ph Meter Titration Of Naoh And Hcl to identify the equivalence point in the titration, we use titration curves and indicators. Determine the equivalence point by. Use of volumetric flask, and graduated pipet. According to the concentration of acid and base solutions, we have. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of. Ph Meter Titration Of Naoh And Hcl.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Ph Meter Titration Of Naoh And Hcl Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Use of volumetric flask, and graduated pipet. you will titrate a solution of hcl with a standardized solution of naoh while measuring the. Ph Meter Titration Of Naoh And Hcl.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Ph Meter Titration Of Naoh And Hcl Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. to identify the equivalence point in the titration, we use titration curves and indicators. Use of volumetric flask, and graduated pipet. Determine the equivalence point by. According to the concentration of. Ph Meter Titration Of Naoh And Hcl.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Ph Meter Titration Of Naoh And Hcl Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. to identify the equivalence point in the titration, we use titration curves and indicators. you will titrate a solution of hcl with a standardized solution of naoh while measuring the. Ph Meter Titration Of Naoh And Hcl.
From www.vrogue.co
The Graph Of Ph During The Titration Of Naoh And Hcl vrogue.co Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00. Ph Meter Titration Of Naoh And Hcl.
From www.vrogue.co
The Graph Of Ph During The Titration Of Naoh And Hcl vrogue.co Ph Meter Titration Of Naoh And Hcl Determine the equivalence point by. Use of volumetric flask, and graduated pipet. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? to identify the equivalence point in the titration, we use titration curves and indicators. Blocked areas on the curve indicate the ph. Ph Meter Titration Of Naoh And Hcl.
From www.researchgate.net
Direct pHmetric titration curves with NaOH (a) and HCl (b) solutions Ph Meter Titration Of Naoh And Hcl Use of volumetric flask, and graduated pipet. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. According to the concentration of acid and base solutions, we have. Determine the equivalence point by. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Blocked. Ph Meter Titration Of Naoh And Hcl.
From ck12.org
Titration CK12 Foundation Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. to identify the equivalence point in the titration, we use titration curves and indicators. Use of volumetric flask, and graduated pipet. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Determine the equivalence. Ph Meter Titration Of Naoh And Hcl.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Ph Meter Titration Of Naoh And Hcl Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. to identify the equivalence point in the titration, we use titration curves and indicators. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Use of volumetric flask,. Ph Meter Titration Of Naoh And Hcl.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Ph Meter Titration Of Naoh And Hcl Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration.. Ph Meter Titration Of Naoh And Hcl.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Use of volumetric flask, and graduated pipet. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Determine the equivalence point by. to identify the equivalence point in. Ph Meter Titration Of Naoh And Hcl.
From www.scribd.com
acidbase_titration_using_ph_meter_and_finding_the_equivalence_point Ph Meter Titration Of Naoh And Hcl to identify the equivalence point in the titration, we use titration curves and indicators. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. According to the concentration of acid and base solutions, we have. Example \(\pageindex{3}\) what is the ph. Ph Meter Titration Of Naoh And Hcl.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Ph Meter Titration Of Naoh And Hcl you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. to identify the equivalence point in the titration, we use titration curves and indicators. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of. Ph Meter Titration Of Naoh And Hcl.
From www.slideserve.com
PPT Determine the pH during the titration of 75.0 mL of 0.100 M HCl Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Determine the equivalence point by. Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. to identify. Ph Meter Titration Of Naoh And Hcl.
From www.slideserve.com
PPT Determine the pH during the titration of 75.0 mL of 0.100 M HCl Ph Meter Titration Of Naoh And Hcl According to the concentration of acid and base solutions, we have. to identify the equivalence point in the titration, we use titration curves and indicators. you will titrate a solution of hcl with a standardized solution of naoh while measuring the ph throughout the course of the titration. Example \(\pageindex{3}\) what is the ph when 48.00 ml of. Ph Meter Titration Of Naoh And Hcl.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Ph Meter Titration Of Naoh And Hcl Blocked areas on the curve indicate the ph range in which phenolphthalein and methyl red change colors. Titration of 25.0 ml of 0.1m hcl by 0.1 m naoh. Use of volumetric flask, and graduated pipet. to identify the equivalence point in the titration, we use titration curves and indicators. Determine the equivalence point by. Example \(\pageindex{3}\) what is the. Ph Meter Titration Of Naoh And Hcl.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Ph Meter Titration Of Naoh And Hcl Determine the equivalence point by. to identify the equivalence point in the titration, we use titration curves and indicators. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Use of volumetric flask, and graduated pipet. Titration of 25.0 ml of 0.1m hcl by. Ph Meter Titration Of Naoh And Hcl.