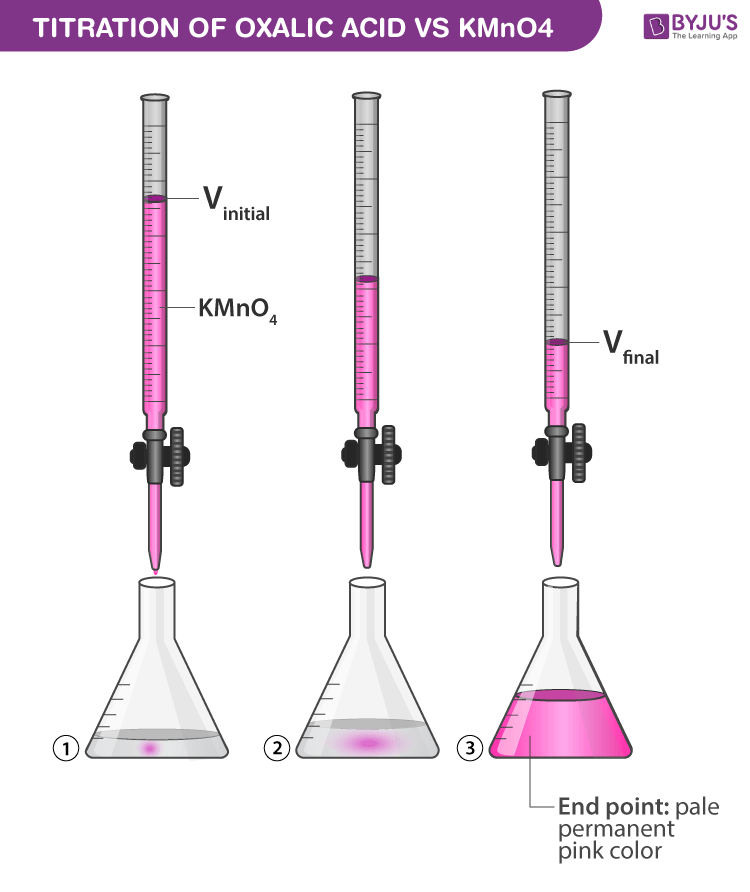
Titration Using Kmno4 . In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Standardization of kmno4(aq) with na2c2o4. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration.
from byjus.com
the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Standardization of kmno4(aq) with na2c2o4. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in.
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12
Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Standardization of kmno4(aq) with na2c2o4.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe. Titration Using Kmno4.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of. Titration Using Kmno4.
From www.scribd.com
Assay of Ferrous Sulphate Using KMnO4 Solution PDF Titration Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as. Titration Using Kmno4.
From www.youtube.com
Chemistry Practical Titration of KMnO4 Solution with Standard Mohrs Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno. Titration Using Kmno4.
From www.studypool.com
SOLUTION Titration mohr s salt vs kmno4 Studypool Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c. Titration Using Kmno4.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4). Titration Using Kmno4.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. Standardization of. Titration Using Kmno4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. Standardization of kmno4(aq) with na2c2o4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the titration of potassium permanganate (kmno 4) against oxalic acid. Titration Using Kmno4.
From www.toppr.com
KMnO4 solution is to be standardized by titration against As2O3 (s) . A Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Standardization of kmno4(aq) with na2c2o4. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine. Titration Using Kmno4.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Titration Using Kmno4 Standardization of kmno4(aq) with na2c2o4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to. Titration Using Kmno4.
From www.chegg.com
Solved Nazl204 KMnO4 Ex. 2 Titration of 0.2121 g of pure Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c. Titration Using Kmno4.
From www.youtube.com
Titration of Oxalic acid vs KMnO4B.Sc 1st sem (NEP)Dr.Chhavi Purwar Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine. Titration Using Kmno4.
From www.youtube.com
Volumetric Titration KMnO4 v/s Mohr's Salt Chemistry Practical Class Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. Standardization of kmno4(aq) with na2c2o4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the titration of potassium permanganate (kmno 4) against mohr salt. Titration Using Kmno4.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment. Titration Using Kmno4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron. Titration Using Kmno4.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Titration Using Kmno4 Standardization of kmno4(aq) with na2c2o4. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4). Titration Using Kmno4.
From www.youtube.com
Titration of KMnO4 vs Ferrous ammonium sulphate Redox titrationHow to Titration Using Kmno4 Standardization of kmno4(aq) with na2c2o4. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2. Titration Using Kmno4.
From www.youtube.com
Volumetric titrations demonstration/ Mohr's salt with potassium Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this. Titration Using Kmno4.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. the redox titration of permanganate ions is commonly used to determine the iron content of. Titration Using Kmno4.
From www.youtube.com
How to prepare KMnO4 solution for titration of class xii calculation Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown. Titration Using Kmno4.
From www.youtube.com
Titration KMnO4 Vs Mohr's Salt Full Experiment + Calculation YouTube Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. the titration of potassium permanganate (kmno 4) against mohr salt is an example of. Titration Using Kmno4.
From www.numerade.com
SOLVEDFor the titration of a KMNO4 solution, Na2C204 is titrated as a Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as. Titration Using Kmno4.
From www.youtube.com
Volumetric TitrationDetermination of Concentration of KMnO4 using Std Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. the titration of potassium permanganate (kmno 4) against mohr salt is an example. Titration Using Kmno4.
From www.congress-intercultural.eu
To Determine The Molarity Of KMnO4 Solution By Titrating It, 44 OFF Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to. Titration Using Kmno4.
From exoyrpuml.blob.core.windows.net
Titration Of Kmno4 With Sodium Oxalate at Mike Stevens blog Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown. Titration Using Kmno4.
From www.youtube.com
class 12 PRACTICAL CHEMISTRY Redox Titration of KMnO4 against Mohr's Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to. Titration Using Kmno4.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o. Titration Using Kmno4.
From www.youtube.com
Titration using acidified KMnO4 and acidified K2Cr2O7 Chemistry Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o. Titration Using Kmno4.
From www.numerade.com
SOLVED The KMnO4 solution is first standardized by titration using Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Standardization of kmno4(aq) with na2c2o4. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in this study, relation between an oxidising agent (oxidant) and reducing. Titration Using Kmno4.
From www.youtube.com
KMnO4 Titration Time Lapse [HD] YouTube Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Standardization of kmno4(aq). Titration Using Kmno4.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment Titration Using Kmno4 in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Standardization of kmno4(aq) with na2c2o4. the redox titration of permanganate ions is commonly used to. Titration Using Kmno4.
From www.youtube.com
Titration1, Prepare M/20 solution of Mohr's salt,Find out the molarity Titration Using Kmno4 the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Standardization of kmno4(aq) with na2c2o4. In this experiment you will use a standard solution of. Titration Using Kmno4.
From www.studypool.com
SOLUTION Titration of kmno4 vs oxalic acid Studypool Titration Using Kmno4 Standardization of kmno4(aq) with na2c2o4. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o. Titration Using Kmno4.
From www.youtube.com
Preparation, Standardization of 0.1N KMnO4 solution YouTube Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. in this study, relation between an oxidising agent (oxidant) and reducing. Titration Using Kmno4.
From www.youtube.com
Titration KMnO4 with Mohr's salt ( chemistry practical) YouTube Titration Using Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. in this study, relation between an oxidising agent (oxidant) and reducing agent (reductant) can be employed in. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as. Titration Using Kmno4.