Medical Devices Europe . Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. There are more than 500,000 products,. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. We represent diagnostics and medical devices manufacturers operating in europe. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
from www.orielstat.com
In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. There are more than 500,000 products,. In the european union (eu) they must undergo a conformity. We represent diagnostics and medical devices manufacturers operating in europe. Medical devices are products or equipment intended for a medical purpose. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on.
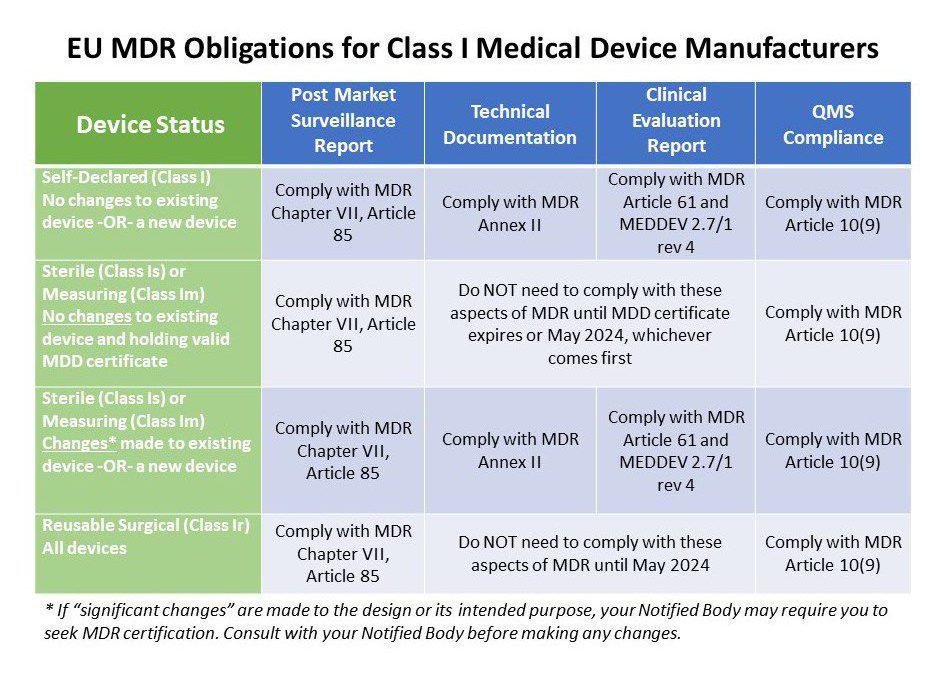
All Class 1 Medical Device Manufacturers Must Meet These Specific EU MDR Requirements Oriel
Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. In the european union (eu) they must undergo a conformity. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Medical devices are products or equipment intended for a medical purpose. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. We represent diagnostics and medical devices manufacturers operating in europe. There are more than 500,000 products,. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Devices Europe There are more than 500,000 products,. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In this guide, we present the different classes for medical. Medical Devices Europe.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and US Regulations Medidee Medical Devices Europe We represent diagnostics and medical devices manufacturers operating in europe. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements. Medical Devices Europe.
From www.medeurope.eu
Med Europe s.r.l. Technologically advanced disposable medical devices Medical Devices Europe Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. There are more than 500,000 products,. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they. Medical Devices Europe.
From www.databridgemarketresearch.com
Europe Smart Medical Devices Market Report Industry Trends and Forecast to 2028 Data Bridge Medical Devices Europe Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and in vitro diagnostics to align. Medical Devices Europe.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Devices Europe Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. Medical devices are products or equipment intended for a medical purpose. We represent diagnostics and medical devices manufacturers operating in europe. In the european. Medical Devices Europe.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Devices Europe In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. We represent diagnostics and medical devices manufacturers operating in europe. Medical devices are products or equipment intended for a medical purpose. There are more than 500,000 products,. In the european union (eu) they must undergo a conformity. Eudamed is the it. Medical Devices Europe.
From www2.deloitte.com
The new European Union medical devices regulation Deloitte Life Sciences and Healthcare Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. There are more than 500,000 products,. We represent diagnostics and medical devices manufacturers operating in europe. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Medical. Medical Devices Europe.
From omcmedical.com
LEGACY MEDICAL DEVICES REQUIREMENTS IN EUROPE OMC Medical Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In the eu, medical devices. Medical Devices Europe.
From twitter.com
IQVIA MedTech on Twitter "Our team is ready to meet with you at Outsourcing in Clinical Trials Medical Devices Europe We represent diagnostics and medical devices manufacturers operating in europe. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. There are more than 500,000 products,. In this guide, we. Medical Devices Europe.
From studylib.net
Comparing Regulation of IVD Medical Devices in Europe and Canada Medical Devices Europe We represent diagnostics and medical devices manufacturers operating in europe. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. There are more than 500,000 products,. Medical devices are products or equipment intended for a medical purpose. In this guide, we present the different classes for medical devices, explain how. Medical Devices Europe.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I more involved Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu revised the laws governing medical devices and in vitro diagnostics to. Medical Devices Europe.
From criticalsoftware.com
MDR 2021 What It Means for Medical Devices in Europe Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In the european union (eu) they must undergo a conformity. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. We represent diagnostics and medical devices manufacturers. Medical Devices Europe.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU MDR Requirements Oriel Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. Eudamed is the it system developed by the european commission to implement. Medical Devices Europe.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Devices Europe There are more than 500,000 products,. We represent diagnostics and medical devices manufacturers operating in europe. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In. Medical Devices Europe.
From www.slideserve.com
PPT Medical Devices In Europe PowerPoint Presentation, free download ID5808782 Medical Devices Europe Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. We represent diagnostics and medical devices manufacturers operating in europe. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and. Medical Devices Europe.
From www.researchandmarkets.com
European Medical Devices Industry Report Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Medical devices are products or equipment intended for a medical purpose. We represent diagnostics and medical devices manufacturers operating in europe. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements. Medical Devices Europe.
From www.scribd.com
Medical Devices For The EU 070910 PDF Medical Device European Union Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on.. Medical Devices Europe.
From credevo.com
Europe Medical Device Market Approval Credevo Articles Medical Devices Europe In the european union (eu) they must undergo a conformity. We represent diagnostics and medical devices manufacturers operating in europe. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20.. Medical Devices Europe.
From pvcmed.org
Why PVC should remain the preferred material in healthcare and elsewhere PVCMed Alliance Medical Devices Europe There are more than 500,000 products,. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. In the european union (eu) they must undergo a conformity. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. We represent diagnostics and. Medical Devices Europe.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. We. Medical Devices Europe.
From nexgoal.com
2022 Trends To Monitor in the Medical Technology Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they. Medical Devices Europe.
From studylib.net
Regulatory Process For Medical Devices In Europe Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. There are more than 500,000 products,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity.. Medical Devices Europe.
From cmsmedtech.com
medical device registration in Europe Medical Devices Europe Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. There are more than 500,000 products,. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. In this guide, we present the different classes for medical devices, explain how medical devices must be classified,. Medical Devices Europe.
From pepgra.com
Preparing For The Future The New European Union Medical Devices Regulation pepgra Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Medical devices are products or equipment intended for a medical purpose. Regulation. Medical Devices Europe.
From twitter.com
IQVIA MedTech on Twitter "Will you be attending Outsourcing in Clinical Trials Medical Devices Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. Medical devices are products or equipment intended for a medical purpose. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. In the eu, medical devices must undergo assessments to. Medical Devices Europe.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. We represent diagnostics and medical devices. Medical Devices Europe.
From www.slideshare.net
MDR Compliance Requirements for Medical Devices in Europe PDF Medical Devices Europe There are more than 500,000 products,. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Medical Devices Europe.
From www.nsmedicaldevices.com
Top 10 European medical device companies in 2020 by total revenue Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. We represent diagnostics and medical devices manufacturers operating in europe. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. There are more than 500,000 products,. Regulation (eu) 2017/745 of the european parliament and of. Medical Devices Europe.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment. Medical Devices Europe.
From www.databridgemarketresearch.com
Europe Wearable Medical Devices Market Report Industry Trends and Forecast to 2027 Data Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. There are more than 500,000 products,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Devices Europe.
From medical-device-manufacturing-europe.manufacturingtechnologyinsights.com
Top Medical Device Manufacturers in Europe medicaldevicemanufacturingeurope Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Eudamed is the it system developed by the european commission to implement regulation (eu) 2017/745 on. There are more than 500,000 products,. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments. Medical Devices Europe.
From blog.zymewire.com
Outsourcing in Clinical Trials (OCT) Medical Devices Europe 2020 Medical Devices Europe The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20. There are more than 500,000 products,. In the eu, medical devices must undergo assessments to demonstrate that they meet legal requirements to ensure they are. Regulation (eu) 2017/745 of the european parliament and of the council. Medical Devices Europe.
From www.youtube.com
European Medical Device Registration Chapter 1 Overview YouTube Medical Devices Europe In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu revised the laws governing medical devices and in vitro diagnostics to align with the. Medical Devices Europe.
From inkwoodresearch.com
Europe Surgical and Medical Device Market Forecast 20172026 Medical Devices Europe There are more than 500,000 products,. We represent diagnostics and medical devices manufacturers operating in europe. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. In the eu, medical devices must undergo assessments to demonstrate that they meet. Medical Devices Europe.
From qservecro.com
Medical Devices Outsourcing In Clinical Trials Medical Devices Europe Medical devices are products or equipment intended for a medical purpose. We represent diagnostics and medical devices manufacturers operating in europe. In the european union (eu) they must undergo a conformity. In this guide, we present the different classes for medical devices, explain how medical devices must be classified, delve into the classification rules, and. The eu revised the laws. Medical Devices Europe.