Discussion On Back Titration Lab Report . The remaining excess reagent is then titrated with another, second reagent. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Calculate analyte concentrations given experimental data from a back titration. Titration is the process of determining the concentration of an unknown solution using a solution of known. This list outlines the tasks in submitting your report for experiment. This resource accompanies the essential guide to teaching quantitative chemistry in education in. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Click the google sheet template and make a copy.
from www.chegg.com
Click the google sheet template and make a copy. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. This list outlines the tasks in submitting your report for experiment. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. The remaining excess reagent is then titrated with another, second reagent. Titration is the process of determining the concentration of an unknown solution using a solution of known. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. This resource accompanies the essential guide to teaching quantitative chemistry in education in. Calculate analyte concentrations given experimental data from a back titration.
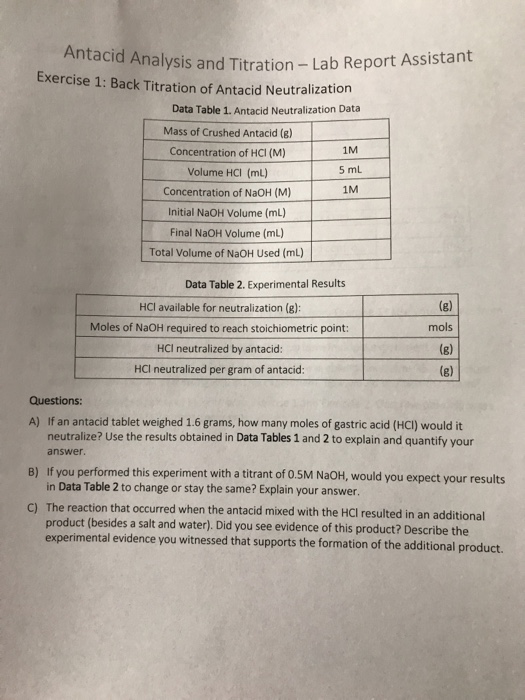
Analysis and Titration Lab Report Assistamt Exercise
Discussion On Back Titration Lab Report Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The remaining excess reagent is then titrated with another, second reagent. Click the google sheet template and make a copy. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Titration is the process of determining the concentration of an unknown solution using a solution of known. Calculate analyte concentrations given experimental data from a back titration. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This resource accompanies the essential guide to teaching quantitative chemistry in education in. This list outlines the tasks in submitting your report for experiment.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. This resource accompanies the essential guide to teaching quantitative chemistry in education in. A back titration is. Discussion On Back Titration Lab Report.
From www.studocu.com
Back Titrationlab4 lab report Determination of Aspirin using Back Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. Click the google sheet template and make a copy. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This list outlines the tasks in submitting your report for experiment. The resulting. Discussion On Back Titration Lab Report.
From www.vrogue.co
Acid Base Titration Calculation Example Youtube vrogue.co Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. Calculate analyte concentrations given experimental data from a back titration. Titration is the process of determining the concentration of an unknown solution using a solution of known. Back titration is used to find the number of moles of a substance by reacting it with an excess volume. Discussion On Back Titration Lab Report.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Discussion On Back Titration Lab Report This list outlines the tasks in submitting your report for experiment. The remaining excess reagent is then titrated with another, second reagent. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Calculate analyte concentrations given experimental data from a back titration. The resulting mixture. Discussion On Back Titration Lab Report.
From studylib.net
Titration Lab Report to be filled in Siverling Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Click the google sheet template and make a copy. Titration. Discussion On Back Titration Lab Report.
From www.scribd.com
chemistry lab report titration lab Molar Concentration Mole (Unit) Discussion On Back Titration Lab Report The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Calculate analyte concentrations given experimental data from a back titration. Click the google sheet template and make a copy. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the. Discussion On Back Titration Lab Report.
From www.chegg.com
Analysis and Titration Lab Report Assistamt Exercise Discussion On Back Titration Lab Report Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The remaining excess reagent is then titrated with another, second reagent. Click the google sheet template and make a copy. This list outlines the tasks in submitting your report for experiment. Calculate analyte concentrations given. Discussion On Back Titration Lab Report.
From www.chegg.com
Solved Antacid Analysis and TitrationLab Report Assistant Discussion On Back Titration Lab Report A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This resource accompanies the essential guide to teaching quantitative chemistry in education in. Click the google sheet template and make a copy. Titration is the process of determining the concentration of an unknown solution using. Discussion On Back Titration Lab Report.
From www.scribd.com
chemistry lab report (back titration) Titration Acid Discussion On Back Titration Lab Report A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Calculate analyte concentrations given experimental data from a back titration. This list outlines the tasks in submitting your report for experiment. Click the google sheet template and make a copy. In a back titration, an. Discussion On Back Titration Lab Report.
From www.vrogue.co
Acid Base Titration Lab Report Answers Stephanie Mack vrogue.co Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The resulting mixture is then titrated to work out the. Discussion On Back Titration Lab Report.
From www.studocu.com
Lab 7 p H and titration lab report Name Discussion On Back Titration Lab Report Titration is the process of determining the concentration of an unknown solution using a solution of known. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The remaining excess reagent is then titrated with another, second reagent. This resource accompanies the essential guide to. Discussion On Back Titration Lab Report.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Discussion On Back Titration Lab Report The resulting mixture is then titrated to work out the number of moles of the reactant in excess. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The remaining excess reagent is then titrated with another, second reagent. Calculate analyte concentrations given experimental data. Discussion On Back Titration Lab Report.
From childhealthpolicy.vumc.org
💣 Discussion lab report acid base titration. Lab Report Acid Base Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Click the google sheet template and make a copy. The remaining excess reagent is then titrated with another, second reagent. A back titration is a titration method where the. Discussion On Back Titration Lab Report.
From www.vrogue.co
Acid Base Titration Lab Dataclassroom Lab Report Titr vrogue.co Discussion On Back Titration Lab Report Calculate analyte concentrations given experimental data from a back titration. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The remaining excess reagent is then titrated with another, second reagent. This list outlines the tasks in submitting your report for experiment. Click the google. Discussion On Back Titration Lab Report.
From nerdyseal.com
Back titration lab report 655 Words NerdySeal Discussion On Back Titration Lab Report Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. This list outlines the tasks in submitting your report for experiment. Titration is the process of determining the concentration of an unknown solution using a solution of known. In a back titration, an excess of. Discussion On Back Titration Lab Report.
From mavink.com
Acid Base Titration Lab Procedure Discussion On Back Titration Lab Report Click the google sheet template and make a copy. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. Titration is the process of determining the concentration. Discussion On Back Titration Lab Report.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Discussion On Back Titration Lab Report A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Calculate analyte concentrations given experimental data from a back titration. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration.. Discussion On Back Titration Lab Report.
From paperap.com
Acid Base Titration Lab Report Discussion Essay Example Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount. Discussion On Back Titration Lab Report.
From www.slideshare.net
Titration experiment report Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. This resource accompanies the essential guide to teaching quantitative chemistry in education in. Calculate analyte concentrations given. Discussion On Back Titration Lab Report.
From www.studocu.com
Back Titration Lab Report Lab Report 4/11/ Determination of Na2CO3 in Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. Titration is the process of determining the concentration of an unknown solution using a solution of known. The remaining excess reagent is then titrated with another, second reagent. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. In. Discussion On Back Titration Lab Report.
From www.scribd.com
titration lab report Titration Ph Discussion On Back Titration Lab Report This list outlines the tasks in submitting your report for experiment. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This resource accompanies the essential guide to teaching quantitative chemistry in education in. Calculate analyte concentrations given experimental data from a back titration. The. Discussion On Back Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Discussion On Back Titration Lab Report Calculate analyte concentrations given experimental data from a back titration. This resource accompanies the essential guide to teaching quantitative chemistry in education in. Click the google sheet template and make a copy. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. This list outlines. Discussion On Back Titration Lab Report.
From www.studypool.com
SOLUTION Titration of vinegar lab report Studypool Discussion On Back Titration Lab Report Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This list outlines the tasks in submitting your report for. Discussion On Back Titration Lab Report.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Discussion On Back Titration Lab Report Calculate analyte concentrations given experimental data from a back titration. Titration is the process of determining the concentration of an unknown solution using a solution of known. This resource accompanies the essential guide to teaching quantitative chemistry in education in. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely. Discussion On Back Titration Lab Report.
From www.slideshare.net
Titration report Discussion On Back Titration Lab Report This list outlines the tasks in submitting your report for experiment. Titration is the process of determining the concentration of an unknown solution using a solution of known. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The resulting mixture is then titrated to. Discussion On Back Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Discussion On Back Titration Lab Report A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This list outlines the tasks in submitting your report for experiment. Click the google sheet template and make a copy. This resource accompanies the essential guide to teaching quantitative chemistry in education in. In a. Discussion On Back Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Discussion On Back Titration Lab Report This list outlines the tasks in submitting your report for experiment. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Click the google sheet template and make a copy. Back titration is used to find the number of moles of a substance by reacting. Discussion On Back Titration Lab Report.
From www.slideshare.net
Chemistry report final Discussion On Back Titration Lab Report The remaining excess reagent is then titrated with another, second reagent. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. This resource accompanies the essential guide to teaching quantitative chemistry in education in. This list outlines the tasks in submitting your report for experiment.. Discussion On Back Titration Lab Report.
From hxezighol.blob.core.windows.net
Oxidation Reduction Titration Lab Report Discussion at Mickie Brown blog Discussion On Back Titration Lab Report A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. This list outlines the tasks in submitting your report for experiment. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary. Discussion On Back Titration Lab Report.
From studymoose.com
AcidBase Titration Experiment Report StudyMoose Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. This resource accompanies the essential guide to teaching quantitative chemistry. Discussion On Back Titration Lab Report.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Discussion On Back Titration Lab Report This resource accompanies the essential guide to teaching quantitative chemistry in education in. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Click the google sheet template and make a copy. The resulting mixture is then titrated to work out the number of moles. Discussion On Back Titration Lab Report.
From hxezighol.blob.core.windows.net
Oxidation Reduction Titration Lab Report Discussion at Mickie Brown blog Discussion On Back Titration Lab Report Titration is the process of determining the concentration of an unknown solution using a solution of known. Calculate analyte concentrations given experimental data from a back titration. In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The remaining excess reagent is then titrated with. Discussion On Back Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Discussion On Back Titration Lab Report In a back titration, an excess of a reagent to be standardized (in this case hcl) is added to completely react with the primary standard. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Titration is the process of determining the concentration of an unknown solution using a solution of known.. Discussion On Back Titration Lab Report.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Discussion On Back Titration Lab Report Calculate analyte concentrations given experimental data from a back titration. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Click the google sheet template and make a copy. This list outlines the tasks in submitting your report for experiment. Back titration is used to. Discussion On Back Titration Lab Report.
From childhealthpolicy.vumc.org
😱 Titration lab report discussion. Lab Report 9. 20221018 Discussion On Back Titration Lab Report Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Titration is the process of determining the concentration of an unknown solution using a solution of known. A back titration is a titration method where the concentration of an analyte is determined by reacting it. Discussion On Back Titration Lab Report.