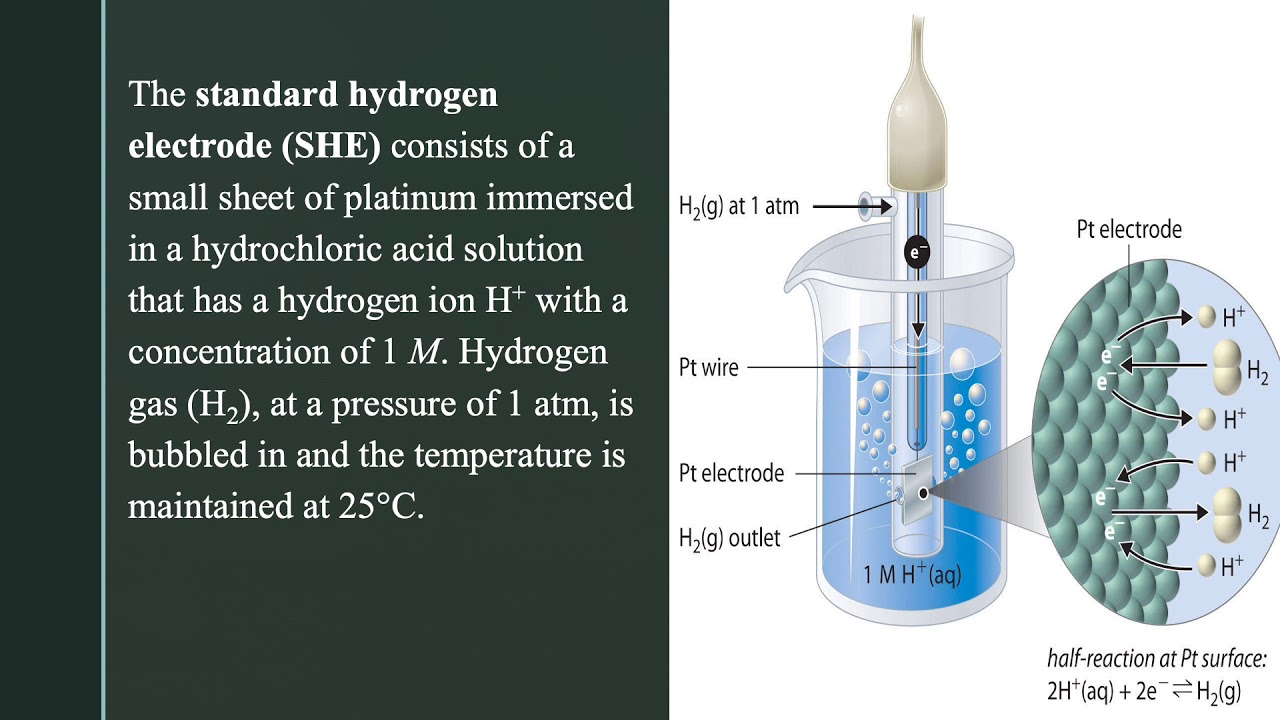
Standard Hydrogen Electrode . Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of
from www.youtube.com
See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed.
Define standard hydrogen electrode SHE and write the reactions that
Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Hydrogen gas in equilibrium with h.
From www.vrogue.co
Ii Draw Well Labeled Diagram Of Standard Hydrogen Ele vrogue.co Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its. Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode See a labelled diagram of she and a solved example problem involving its potential. Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is. Standard Hydrogen Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. See a labelled diagram of she and a solved example problem involving its potential. Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is. Standard Hydrogen Electrode.
From slideplayer.com
Advanced Higher Chemistry Unit 2(e) ppt download Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of. Standard Hydrogen Electrode.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Standard Hydrogen Electrode See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is used as a reference. Standard Hydrogen Electrode.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the. Standard Hydrogen Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Although the standard hydrogen electrode is one of the most fundamental concepts of. Standard Hydrogen Electrode.
From www.slideserve.com
PPT STANDARD REDUCTION POTENTIAL PowerPoint Presentation, free Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen. Standard Hydrogen Electrode.
From mungfali.com
Hydrogen Electrode Diagram Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any. Standard Hydrogen Electrode.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode. Standard Hydrogen Electrode.
From www.shutterstock.com
Standard Hydrogen Electrode Reference Electrode vector de stock (libre Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated. Standard Hydrogen Electrode.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Standard Hydrogen Electrode See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn. Standard Hydrogen Electrode.
From slideplayer.com
KNOCKHARDY PUBLISHING ppt download Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any. Standard Hydrogen Electrode.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is. Standard Hydrogen Electrode.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the. Standard Hydrogen Electrode.
From slideplayer.com
Voltammetry and Polarography ppt download Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of See a labelled diagram of she and a solved example problem involving its potential. Learn. Standard Hydrogen Electrode.
From 2012books.lardbucket.org
Electrochemistry Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its. Standard Hydrogen Electrode.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its potential. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is. Standard Hydrogen Electrode.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined. Standard Hydrogen Electrode.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated. Standard Hydrogen Electrode.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Standard Hydrogen Electrode See a labelled diagram of she and a solved example problem involving its potential. Hydrogen gas in equilibrium with h. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is. Standard Hydrogen Electrode.
From app.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any. Standard Hydrogen Electrode.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference. Standard Hydrogen Electrode.
From www.sciencephoto.com
Hydrogen electrode apparatus Stock Image C011/4570 Science Photo Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of. Standard Hydrogen Electrode.
From mavink.com
Hydrogen Electrode Diagram Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed.. Standard Hydrogen Electrode.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. See a labelled diagram of she and a solved example problem involving its potential. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Although the standard hydrogen electrode. Standard Hydrogen Electrode.
From www.shutterstock.com
Diagram Standard Hydrogen Electrode Stock Vector (Royalty Free Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. See a labelled diagram of she and a solved example problem involving its potential. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is. Standard Hydrogen Electrode.
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. See a labelled diagram of she and a solved example problem involving its. Standard Hydrogen Electrode.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of See a labelled diagram of she and a solved example problem involving its potential. Learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode.
From slideplayer.com
Electrochemistry. ppt download Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is constructed. Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its potential. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is. Standard Hydrogen Electrode.
From www.vrogue.co
Ii Draw Well Labeled Diagram Of Standard Hydrogen Ele vrogue.co Standard Hydrogen Electrode Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn. Standard Hydrogen Electrode.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Hydrogen gas in equilibrium with h. See a labelled diagram of she and a solved example problem involving its potential. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Learn what a standard hydrogen electrode (she) is, how it is used as a reference electrode, and how it is. Standard Hydrogen Electrode.
From www.vrogue.co
Ii Draw Well Labeled Diagram Of Standard Hydrogen Ele vrogue.co Standard Hydrogen Electrode See a labelled diagram of she and a solved example problem involving its potential. Learn about the standard hydrogen electrode (she), a reference electrode with a potential of 0 volts, and other types of. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined. Standard Hydrogen Electrode.