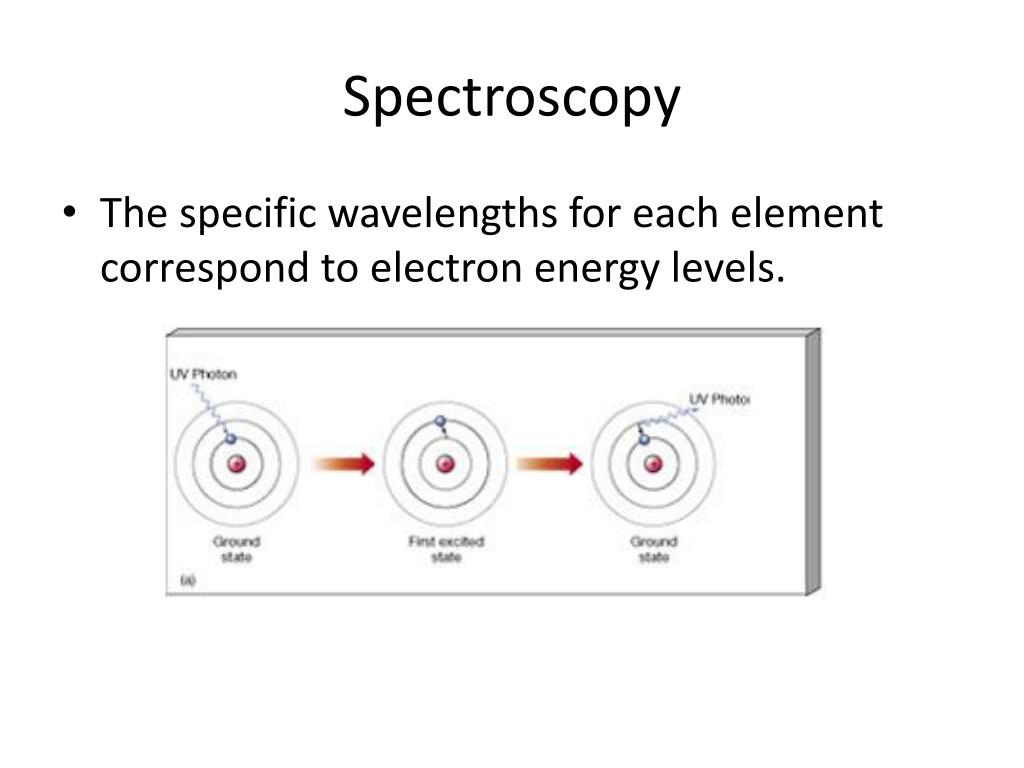
Electronic Energy Level In Spectroscopy . Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. The translational energy levels of a molecule are usually taken to. Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Molecular energy levels and spectroscopy. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The energy level differences are usually high enough that it falls into the. Electronic spectroscopy relies on the quantized nature of energy states.
from www.slideserve.com
Given enough energy, an electron can be excited from. Molecular energy levels and spectroscopy. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. A photon is absorbed (lost) as the molecule is raised to a higher energy level. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The translational energy levels of a molecule are usually taken to. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions.
PPT Light and Matter PowerPoint Presentation, free download ID1588682
Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually high enough that it falls into the. Electronic spectroscopy relies on the quantized nature of energy states. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The translational energy levels of a molecule are usually taken to. Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Molecular energy levels and spectroscopy.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. Given enough energy, an. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Atomic UVVisible Spectroscopy PowerPoint Presentation ID2707921 Electronic Energy Level In Spectroscopy In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually high enough that it falls into the. You will use your spectra for chemical identification, study. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Xray emission spectra and energy level diagrams of the absorption and... Download Scientific Electronic Energy Level In Spectroscopy Molecular energy levels and spectroscopy. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. A photon is absorbed (lost) as the molecule is raised to a higher energy level. The translational energy levels of a molecule. Electronic Energy Level In Spectroscopy.
From www.animalia-life.club
Spectrum Wavelengths Chart Electronic Energy Level In Spectroscopy Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. In this chapter, we begin. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Atomic UVVisible Spectroscopy PowerPoint Presentation ID2707921 Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. A. Electronic Energy Level In Spectroscopy.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. Molecular energy levels and spectroscopy. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences. Electronic Energy Level In Spectroscopy.
From byjus.com
Spectral Series Explained along with Hydrogen spectrum, Rydberg formula. Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The translational energy levels of a molecule are usually taken to. Given enough energy, an electron can be excited from. Molecules can also undergo changes in electronic transitions during microwave and infrared. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Lecture 36 Electronic spectroscopy PowerPoint Presentation, free download ID2487112 Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The energy level differences are usually high enough that it falls into the. Given enough energy, an electron can be excited from. Molecular. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Light and Matter PowerPoint Presentation, free download ID1588682 Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. Molecular energy levels and spectroscopy. A photon is absorbed (lost) as the molecule is raised to a higher energy level. In this chapter, we begin with a discussion of the basic principles of. Electronic Energy Level In Spectroscopy.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. A photon is absorbed (lost) as the molecule is raised to a higher. Electronic Energy Level In Spectroscopy.
From www.renishaw.co.kr
Raman spectroscopy in more detail Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. The energy level differences are usually high enough that it falls into the. Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the. Electronic Energy Level In Spectroscopy.
From www.scribd.com
Molecular Electronic Spectroscopy Energy Level Molecular Orbital Electronic Energy Level In Spectroscopy The energy level differences are usually high enough that it falls into the. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. Electronic spectroscopy relies on the quantized nature of energy states. The translational energy levels of a molecule are usually taken to. In this chapter, we begin with a discussion of the basic principles of. Electronic Energy Level In Spectroscopy.
From www.globalsino.com
Introduction of EELS Spectrum (Electron Energy Loss Spectroscopy) Electronic Energy Level In Spectroscopy The translational energy levels of a molecule are usually taken to. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. You. Electronic Energy Level In Spectroscopy.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. The energy level differences are usually high enough that it falls into the. Electronic spectroscopy relies on the quantized nature of energy states. Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the quantized nature of energy states. The translational energy levels of a molecule are usually taken to. In this. Electronic Energy Level In Spectroscopy.
From learnbin.net
UVVis Spectroscopy Fundamentals Of UV Visible Spectroscopy Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually high enough that it falls into the. Given enough energy, an electron can be excited from. Molecular energy levels and spectroscopy. The translational energy levels of a molecule are usually taken to. Given enough energy, an electron can be excited from. Molecules can also. Electronic Energy Level In Spectroscopy.
From ar.inspiredpencil.com
Spectrum Energy Levels Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. A photon is absorbed (lost) as the molecule is raised to a higher energy level. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Revealing the surface chemistry by electron energy loss spectroscopy... Download Scientific Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. A photon is absorbed (lost) as the molecule is raised to a higher energy level. The translational energy levels of a molecule are usually taken to. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Spectroscopic Analysis Part 4 Molecular Energy Levels and IR Spectroscopy PowerPoint Electronic Energy Level In Spectroscopy A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. The translational. Electronic Energy Level In Spectroscopy.
From www.globalsino.com
Introduction of EELS Spectrum (Electron Energy Loss Spectroscopy) Electronic Energy Level In Spectroscopy A photon is absorbed (lost) as the molecule is raised to a higher energy level. Given enough energy, an electron can be excited from. The energy level differences are usually high enough that it falls into the. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. You will use your. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Typical diagram of the electronic energy levels of a molecule with... Download Scientific Diagram Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The translational energy levels of a molecule are usually taken to. Given enough energy, an electron can be excited from. Molecules can. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Atomic UVVisible Spectroscopy PowerPoint Presentation ID2707921 Electronic Energy Level In Spectroscopy Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions.. Electronic Energy Level In Spectroscopy.
From chem.libretexts.org
Raman spectroscopy Chemistry LibreTexts Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. The energy level differences are usually high enough that it falls into the. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic. Electronic Energy Level In Spectroscopy.
From chem.libretexts.org
1.8 The Bohr Theory of the Hydrogen Atom Chemistry LibreTexts Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Molecular energy levels and spectroscopy. The translational energy levels of a molecule are usually taken to. In this chapter, we begin with a. Electronic Energy Level In Spectroscopy.
From www.nagwa.com
Question Video Identifying Electron Energy Level Transitions of the Hydrogen Emission Spectrum Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Electronic spectroscopy relies on the quantized nature of energy states. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be. Electronic Energy Level In Spectroscopy.
From socratic.org
What must happen for an electron to move to a higher energy level? Socratic Electronic Energy Level In Spectroscopy In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. The translational energy levels of a molecule are usually taken to. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually high enough that it falls into the. Given enough energy,. Electronic Energy Level In Spectroscopy.
From stock.adobe.com
chart of electron configuration with each energy level for element in chemistry. Stock Electronic Energy Level In Spectroscopy In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Molecular energy levels and spectroscopy. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. The energy level differences are usually high enough that it falls into the. Electronic. Electronic Energy Level In Spectroscopy.
From www.chemistryviews.org
The Spectrum ChemistryViews Electronic Energy Level In Spectroscopy A photon is absorbed (lost) as the molecule is raised to a higher energy level. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Molecular energy levels and spectroscopy. The energy level differences are usually high enough that it falls into the. Electronic spectroscopy relies on the quantized nature of energy. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Energy level diagram showing electron transitions producing Fe K and L... Download Scientific Electronic Energy Level In Spectroscopy A photon is absorbed (lost) as the molecule is raised to a higher energy level. Molecular energy levels and spectroscopy. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Electronic spectroscopy relies on the quantized nature of energy states. Molecules can also undergo changes in electronic transitions during microwave and infrared. Electronic Energy Level In Spectroscopy.
From byjus.com
The energy levels of the hydrogen spectrum is shown in the figure. There are some transitions A Electronic Energy Level In Spectroscopy The translational energy levels of a molecule are usually taken to. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Energy level and doublesided Feynman diagrams of the IR absorption,... Download Scientific Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Electronic spectroscopy relies on the quantized nature of energy states. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. The energy level differences are usually high enough that it falls into the.. Electronic Energy Level In Spectroscopy.
From www.masterorganicchemistry.com
Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model Electronic Energy Level In Spectroscopy Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. Given enough energy, an electron can be excited from. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. The translational energy levels of a molecule are usually taken to.. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT Electronic Spectroscopy of molecules PowerPoint Presentation, free download ID3213698 Electronic Energy Level In Spectroscopy The energy level differences are usually high enough that it falls into the. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. Molecules can also undergo changes in electronic transitions during microwave and infrared absorptions. Molecular energy levels and spectroscopy. Electronic spectroscopy. Electronic Energy Level In Spectroscopy.
From www.youtube.com
Atomic spectra Evidence for the quantized Electronic Energy Levels (Structure of atom) YouTube Electronic Energy Level In Spectroscopy You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. The energy level differences are usually high enough that it falls into the. Given enough energy, an electron can be excited from. Molecular energy levels and spectroscopy. Electronic spectroscopy relies on the quantized. Electronic Energy Level In Spectroscopy.
From www.slideserve.com
PPT UltravioletVisible Spectroscopy PowerPoint Presentation, free download ID4329312 Electronic Energy Level In Spectroscopy Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. The energy level differences are usually high enough that it falls into the. A photon is absorbed (lost) as the molecule is raised to a higher energy level. Molecules can also undergo changes in electronic transitions during microwave and infrared. Electronic Energy Level In Spectroscopy.
From www.researchgate.net
Electronic, vibrational, and rotational energy levels for the... Download Scientific Diagram Electronic Energy Level In Spectroscopy Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. You will use your spectra for chemical identification, study of electronic properties of organic molecules and semiconductor quantum. Given enough energy, an electron can be excited from. The. Electronic Energy Level In Spectroscopy.