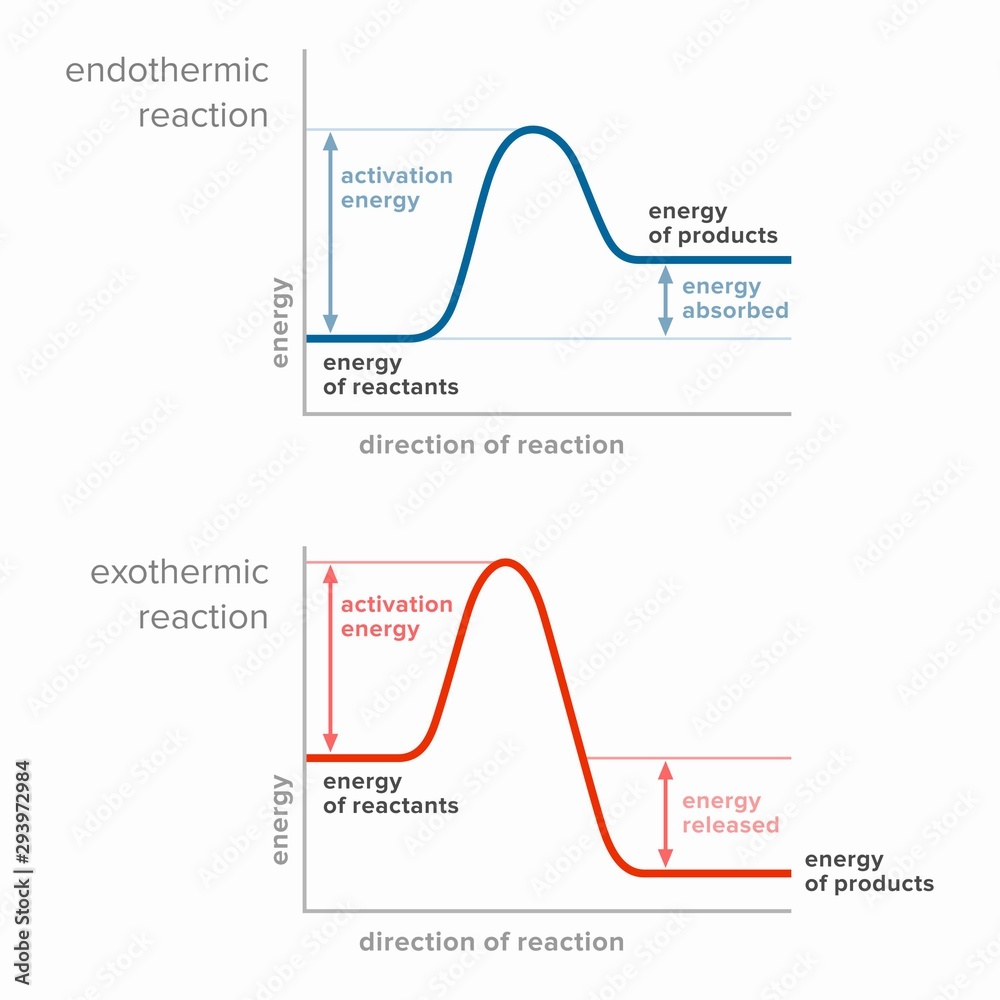
Endo Exothermic Process . It is a reaction that releases energy to its surroundings. Are there any instances when it would be useful to quickly make something hot or cold? Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. Explain how it is useful. The quantity of heat for a process is represented by the letter \(q\). Chemical processes are labeled as exothermic or endothermic. State the law of conservation of energy. Atoms are held together by a certain amount of energy called bond energy. The sign of \(q\) for an endothermic process is positive because the system is gaining heat.
from stock.adobe.com
Define endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Are there any instances when it would be useful to quickly make something hot or cold? Describe how heat is transferred in endothermic and exothermic reactions. Chemical processes are labeled as exothermic or endothermic. Explain how it is useful. The quantity of heat for a process is represented by the letter \(q\). It is a reaction that releases energy to its surroundings. State the law of conservation of energy.
Activation energy in endothermic and exothermic reactions. 素材庫插圖
Endo Exothermic Process Explain how it is useful. Explain how it is useful. Chemical processes are labeled as exothermic or endothermic. State the law of conservation of energy. It is a reaction that releases energy to its surroundings. Are there any instances when it would be useful to quickly make something hot or cold? Describe how heat is transferred in endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. The quantity of heat for a process is represented by the letter \(q\). Define endothermic and exothermic reactions.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs Endo Exothermic Process Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. Are there any instances when it would be useful to quickly make something hot or cold? The quantity of heat for a process is represented by the letter \(q\). Chemical processes are labeled as exothermic or endothermic. Atoms are held together by a certain amount. Endo Exothermic Process.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endo Exothermic Process Chemical processes are labeled as exothermic or endothermic. The quantity of heat for a process is represented by the letter \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Atoms are held together by a certain amount of energy called bond energy. Describe how heat is transferred in endothermic and exothermic reactions.. Endo Exothermic Process.
From mavink.com
Exothermic Reaction Endo Exothermic Process Explain how it is useful. Describe how heat is transferred in endothermic and exothermic reactions. State the law of conservation of energy. Atoms are held together by a certain amount of energy called bond energy. Are there any instances when it would be useful to quickly make something hot or cold? The quantity of heat for a process is represented. Endo Exothermic Process.
From wordmint.com
Endothermic & Exothermic Reactions Crossword WordMint Endo Exothermic Process It is a reaction that releases energy to its surroundings. The quantity of heat for a process is represented by the letter \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Describe how heat is transferred in endothermic and exothermic reactions. State the law of conservation of energy. Atoms are held together. Endo Exothermic Process.
From quizizz.com
Endothermic & Exothermic Chemistry Quiz Quizizz Endo Exothermic Process Explain how it is useful. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. Are there any instances when it would be useful to quickly make something hot or cold? The quantity of heat for a process is. Endo Exothermic Process.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endo Exothermic Process Are there any instances when it would be useful to quickly make something hot or cold? Chemical processes are labeled as exothermic or endothermic. Explain how it is useful. State the law of conservation of energy. Describe how heat is transferred in endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. The. Endo Exothermic Process.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endo Exothermic Process Explain how it is useful. State the law of conservation of energy. Are there any instances when it would be useful to quickly make something hot or cold? Atoms are held together by a certain amount of energy called bond energy. It is a reaction that releases energy to its surroundings. The sign of \(q\) for an endothermic process is. Endo Exothermic Process.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endo Exothermic Process Atoms are held together by a certain amount of energy called bond energy. Define endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Chemical processes are labeled as exothermic or endothermic. Are there any instances when it would be useful to quickly make something hot or cold? It is. Endo Exothermic Process.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endo Exothermic Process Describe how heat is transferred in endothermic and exothermic reactions. Explain how it is useful. Define endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. Are there any instances when it would be useful to quickly make something hot or cold? It is a reaction that releases energy to its surroundings. State. Endo Exothermic Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endo Exothermic Process Define endothermic and exothermic reactions. Are there any instances when it would be useful to quickly make something hot or cold? State the law of conservation of energy. Chemical processes are labeled as exothermic or endothermic. It is a reaction that releases energy to its surroundings. Describe how heat is transferred in endothermic and exothermic reactions. The sign of \(q\). Endo Exothermic Process.
From mungfali.com
Exothermic Vs Endothermic Diagram Endo Exothermic Process Explain how it is useful. Chemical processes are labeled as exothermic or endothermic. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Are there any instances when it would be useful to quickly make something hot or cold? State the law of conservation of energy. It is a reaction that releases energy to. Endo Exothermic Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endo Exothermic Process Define endothermic and exothermic reactions. Chemical processes are labeled as exothermic or endothermic. Atoms are held together by a certain amount of energy called bond energy. Describe how heat is transferred in endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. It is a reaction that releases energy to. Endo Exothermic Process.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Endo Exothermic Process State the law of conservation of energy. Describe how heat is transferred in endothermic and exothermic reactions. Explain how it is useful. Atoms are held together by a certain amount of energy called bond energy. It is a reaction that releases energy to its surroundings. Chemical processes are labeled as exothermic or endothermic. The quantity of heat for a process. Endo Exothermic Process.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endo Exothermic Process Atoms are held together by a certain amount of energy called bond energy. It is a reaction that releases energy to its surroundings. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Chemical processes are labeled as exothermic or endothermic. State the law of conservation of energy. The quantity of heat for a. Endo Exothermic Process.
From www.thoughtco.com
Endothermic Reaction Examples Endo Exothermic Process The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Are there any instances when it would be useful to quickly make something hot or cold? Chemical processes are labeled as exothermic or endothermic. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. State the law of conservation. Endo Exothermic Process.
From fatimakruwgamble.blogspot.com
Examples of Endothermic and Exothermic Reactions FatimakruwGamble Endo Exothermic Process Describe how heat is transferred in endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. Are there any instances when it would be useful to quickly make something hot or cold? Define endothermic and exothermic reactions. Explain how it is useful. State the law of conservation of energy. Chemical processes are labeled. Endo Exothermic Process.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endo Exothermic Process Describe how heat is transferred in endothermic and exothermic reactions. Explain how it is useful. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. The quantity of heat for a process is represented by the letter \(q\). State the law of conservation of energy. Are there any instances when it would be useful. Endo Exothermic Process.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endo Exothermic Process State the law of conservation of energy. Atoms are held together by a certain amount of energy called bond energy. Are there any instances when it would be useful to quickly make something hot or cold? Define endothermic and exothermic reactions. It is a reaction that releases energy to its surroundings. The quantity of heat for a process is represented. Endo Exothermic Process.
From www.pinterest.it
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endo Exothermic Process Define endothermic and exothermic reactions. Chemical processes are labeled as exothermic or endothermic. It is a reaction that releases energy to its surroundings. The quantity of heat for a process is represented by the letter \(q\). Explain how it is useful. Atoms are held together by a certain amount of energy called bond energy. Are there any instances when it. Endo Exothermic Process.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endo Exothermic Process The quantity of heat for a process is represented by the letter \(q\). Describe how heat is transferred in endothermic and exothermic reactions. Chemical processes are labeled as exothermic or endothermic. State the law of conservation of energy. It is a reaction that releases energy to its surroundings. Are there any instances when it would be useful to quickly make. Endo Exothermic Process.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endo Exothermic Process Are there any instances when it would be useful to quickly make something hot or cold? Atoms are held together by a certain amount of energy called bond energy. Explain how it is useful. The quantity of heat for a process is represented by the letter \(q\). State the law of conservation of energy. It is a reaction that releases. Endo Exothermic Process.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endo Exothermic Process Explain how it is useful. Atoms are held together by a certain amount of energy called bond energy. Describe how heat is transferred in endothermic and exothermic reactions. State the law of conservation of energy. Chemical processes are labeled as exothermic or endothermic. Define endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the. Endo Exothermic Process.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Endo Exothermic Process State the law of conservation of energy. Describe how heat is transferred in endothermic and exothermic reactions. Explain how it is useful. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. It is a reaction that releases energy to its surroundings. Define endothermic and exothermic reactions. The quantity of heat for a process. Endo Exothermic Process.
From www.vrogue.co
Exothermic Vs Endothermic Reaction Graphs Energy Acti vrogue.co Endo Exothermic Process The quantity of heat for a process is represented by the letter \(q\). Describe how heat is transferred in endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Atoms are held together by a certain amount of energy called bond energy. It is a reaction that releases energy to. Endo Exothermic Process.
From mavink.com
Exothermic Reaction Class 10 Endo Exothermic Process Describe how heat is transferred in endothermic and exothermic reactions. Are there any instances when it would be useful to quickly make something hot or cold? It is a reaction that releases energy to its surroundings. Explain how it is useful. Chemical processes are labeled as exothermic or endothermic. Define endothermic and exothermic reactions. State the law of conservation of. Endo Exothermic Process.
From worksheets.decoomo.com
10++ Endothermic Reactions Vs Exothermic Reactions Worksheet Endo Exothermic Process Atoms are held together by a certain amount of energy called bond energy. Describe how heat is transferred in endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Chemical processes are labeled as exothermic or endothermic. Explain how it is useful. Define endothermic and exothermic reactions. Are there any. Endo Exothermic Process.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. 素材庫插圖 Endo Exothermic Process Chemical processes are labeled as exothermic or endothermic. Define endothermic and exothermic reactions. It is a reaction that releases energy to its surroundings. Explain how it is useful. The quantity of heat for a process is represented by the letter \(q\). Describe how heat is transferred in endothermic and exothermic reactions. Are there any instances when it would be useful. Endo Exothermic Process.
From brainly.in
difference between exothermic and endothermic reaction Brainly.in Endo Exothermic Process Explain how it is useful. Are there any instances when it would be useful to quickly make something hot or cold? The quantity of heat for a process is represented by the letter \(q\). It is a reaction that releases energy to its surroundings. Define endothermic and exothermic reactions. Chemical processes are labeled as exothermic or endothermic. Atoms are held. Endo Exothermic Process.
From quizzcampusbarth.z19.web.core.windows.net
Endothermic Reactions Vs Exothermic Reactions Worksheet Answ Endo Exothermic Process Define endothermic and exothermic reactions. Explain how it is useful. Are there any instances when it would be useful to quickly make something hot or cold? It is a reaction that releases energy to its surroundings. The quantity of heat for a process is represented by the letter \(q\). State the law of conservation of energy. The sign of \(q\). Endo Exothermic Process.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic Endo Exothermic Process State the law of conservation of energy. Describe how heat is transferred in endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. It is a reaction that releases energy to its surroundings. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter \(q\). Chemical processes. Endo Exothermic Process.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Endo Exothermic Process It is a reaction that releases energy to its surroundings. Are there any instances when it would be useful to quickly make something hot or cold? Explain how it is useful. Chemical processes are labeled as exothermic or endothermic. Define endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat.. Endo Exothermic Process.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endo Exothermic Process It is a reaction that releases energy to its surroundings. Define endothermic and exothermic reactions. Atoms are held together by a certain amount of energy called bond energy. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Describe how heat is transferred in endothermic and exothermic reactions. State the law of conservation of. Endo Exothermic Process.
From www.aiophotoz.com
What Is The Difference Between Exothermic And Endothermic Chemical Endo Exothermic Process Chemical processes are labeled as exothermic or endothermic. State the law of conservation of energy. Atoms are held together by a certain amount of energy called bond energy. Explain how it is useful. Are there any instances when it would be useful to quickly make something hot or cold? It is a reaction that releases energy to its surroundings. The. Endo Exothermic Process.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endo Exothermic Process Define endothermic and exothermic reactions. The sign of \(q\) for an endothermic process is positive because the system is gaining heat. Explain how it is useful. Chemical processes are labeled as exothermic or endothermic. It is a reaction that releases energy to its surroundings. The quantity of heat for a process is represented by the letter \(q\). Atoms are held. Endo Exothermic Process.
From circuitlistsememes55.z13.web.core.windows.net
Energy Diagram For An Exothermic Reaction Endo Exothermic Process It is a reaction that releases energy to its surroundings. Are there any instances when it would be useful to quickly make something hot or cold? Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter \(q\). State the law of conservation of. Endo Exothermic Process.