Heat Capacity Coefficients . The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Define heat capacity of an ideal gas for a specific process; Explain the difference between the heat. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Yaws, c.l., chemical properties handbook: It is therefore an extensive property —its value is proportional to. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. These occur at temperatures where the substance undergoes phase. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat.
from www.numerade.com
Define heat capacity of an ideal gas for a specific process; It is therefore an extensive property —its value is proportional to. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. These occur at temperatures where the substance undergoes phase. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Yaws, c.l., chemical properties handbook: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Explain the difference between the heat.
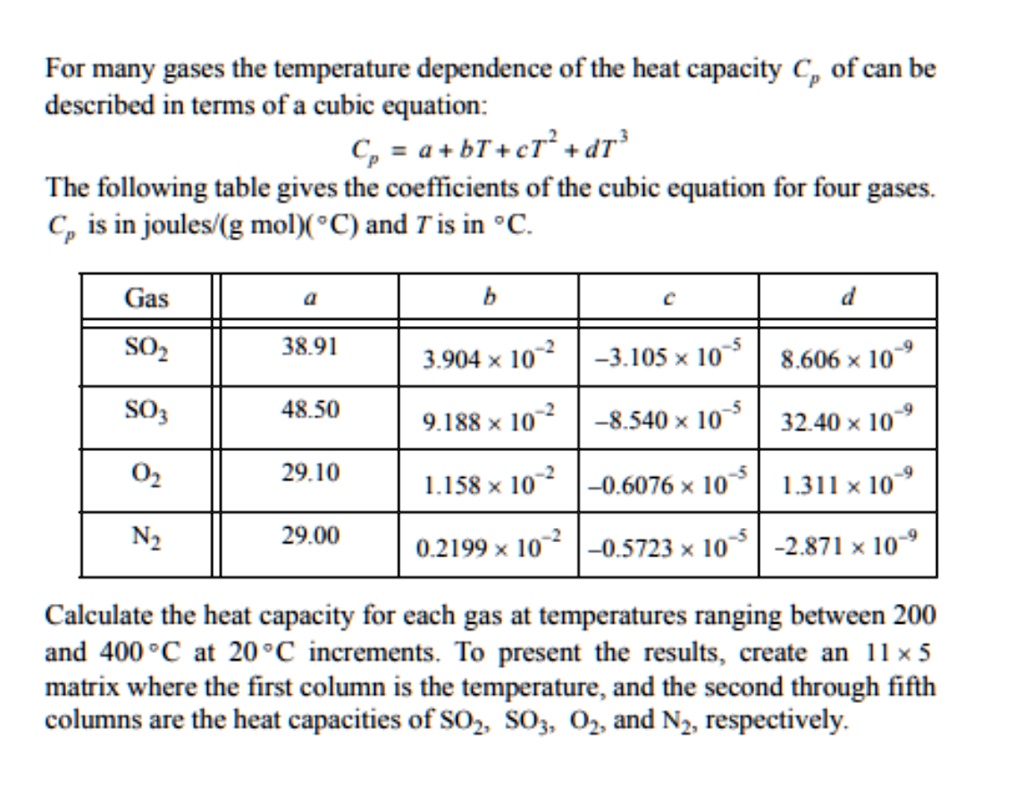
SOLVED For many gases, the temperature dependence of the heat capacity
Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. It is therefore an extensive property —its value is proportional to. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Define heat capacity of an ideal gas for a specific process; In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. These occur at temperatures where the substance undergoes phase. Yaws, c.l., chemical properties handbook: Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Explain the difference between the heat. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities.
From mavink.com
Heat Transfer Coefficient Steel Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Explain the difference between the heat. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. It is therefore an extensive property —its value is proportional to. Define heat. Heat Capacity Coefficients.
From www.chegg.com
14.4 Computing Gas Heat Capacity 11 There are Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Explain the difference between the heat. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is determined by both the type and amount of substance that absorbs or. Heat Capacity Coefficients.
From chempedia.info
Heat Capacities of Gases in the Ideal Gas State Big Chemical Encyclopedia Heat Capacity Coefficients Yaws, c.l., chemical properties handbook: Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. 55. Heat Capacity Coefficients.
From www.chegg.com
Solved Required information For steady flow in a heat Heat Capacity Coefficients Explain the difference between the heat. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. It is therefore an extensive property —its value is proportional to. These occur at temperatures where the substance undergoes phase. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that. Heat Capacity Coefficients.
From www.chegg.com
Solved Table B.2 Heat Capacities Form 1 C1kJ (mol·°C)] or Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; It is therefore an extensive property —its value is proportional to. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. These occur at temperatures where the substance undergoes phase. Define. Heat Capacity Coefficients.
From www.researchgate.net
Liquid Heat Capacity of Alkylene Carbonates Download Table Heat Capacity Coefficients These occur at temperatures where the substance undergoes phase. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Define heat capacity of an ideal gas for a specific process; Yaws, c.l., chemical properties handbook: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific. Heat Capacity Coefficients.
From www.researchgate.net
Variations of the isobaric specific heat capacity c p of water vapor Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; It is therefore an extensive property —its value is proportional to. Explain the difference between the heat. These occur at temperatures where the substance undergoes phase. Define heat capacity of an ideal gas for a specific process; The heat capacity is a smooth, continuous function. Heat Capacity Coefficients.
From www.chegg.com
Solved Calculate the constant pressure and constant volume Heat Capacity Coefficients It is therefore an extensive property —its value is proportional to. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; These occur at temperatures where the substance undergoes phase. Define. Heat Capacity Coefficients.
From www.semanticscholar.org
[PDF] Dependence of the isobaric specific heat capacity of water vapor Heat Capacity Coefficients The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. In thermodynamics,. Heat Capacity Coefficients.
From pharmacalc.blogspot.com
Overall Heat Transfer CoEfficient Calculation Pharma Engineering Heat Capacity Coefficients In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. These occur at temperatures where the. Heat Capacity Coefficients.
From ijtech.eng.ui.ac.id
Journal Issue Heat Capacity Coefficients Define heat capacity of an ideal gas for a specific process; 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. It is therefore an extensive property —its value is proportional to. Explain the difference between the heat. Yaws, c.l., chemical properties handbook: Calculate the specific. Heat Capacity Coefficients.
From www.pinterest.com
coefficient of linear expansion Google Search Thermal, Movement Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Yaws, c.l., chemical properties handbook: Heat capacity is determined by both the type and amount of substance that absorbs. Heat Capacity Coefficients.
From festivaleducazionejesi.com
Specific Heat Of Copper J Kg K / Specific Heat แปลว่าอะไร ดูความหมาย Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Yaws, c.l., chemical properties handbook: Define heat capacity of an ideal gas for a specific process; The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. These occur at. Heat Capacity Coefficients.
From mungfali.com
Table Of Heat Capacity Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. These occur at temperatures where the substance undergoes phase. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. Heat Capacity Coefficients.
From www.linkedin.com
Is specific heat the same as entropy? Why both have the same SI unit kJ Heat Capacity Coefficients The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Explain the difference between the heat. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added. Heat Capacity Coefficients.
From www.oreilly.com
Appendix E. Themodynamic Properties Introductory Chemical Engineering Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. It is therefore an extensive property —its value is proportional to. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Define heat capacity of an ideal gas for a specific. Heat Capacity Coefficients.
From www.chegg.com
Solved TABLE A2 Idealgas specific heats of various common Heat Capacity Coefficients Yaws, c.l., chemical properties handbook: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. It is therefore an extensive property —its value is proportional to. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. The heat capacity. Heat Capacity Coefficients.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas Heat Capacity Coefficients It is therefore an extensive property —its value is proportional to. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. 55 rows the table of specific heat capacities gives the volumetric heat. Heat Capacity Coefficients.
From www.researchgate.net
Temperature dependent coefficients of ideal gas heat capacity equation Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Explain the difference between the heat. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Define heat capacity of an ideal gas for a specific process; These occur at temperatures. Heat Capacity Coefficients.
From www.chegg.com
Solved a) What is the heat capacity of liquid nhexane at Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Yaws, c.l., chemical properties handbook: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Heat capacity is. Heat Capacity Coefficients.
From www.researchgate.net
Lowtemperature heat capacity of MgPd 2 Sb. (a) Equal entropy Heat Capacity Coefficients Explain the difference between the heat. It is therefore an extensive property —its value is proportional to. Define heat capacity of an ideal gas for a specific process; These occur at temperatures where the substance undergoes phase. Yaws, c.l., chemical properties handbook: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific. Heat Capacity Coefficients.
From www.researchgate.net
Measured thermal conductivity, specific heat capacity, diffusivity and Heat Capacity Coefficients These occur at temperatures where the substance undergoes phase. It is therefore an extensive property —its value is proportional to. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. 55 rows the. Heat Capacity Coefficients.
From www.researchgate.net
Specific heat capacity correlation coefficients related to ideal gas Heat Capacity Coefficients Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. These occur at temperatures where the substance undergoes phase. Explain the difference between the heat. Define heat capacity of an ideal gas for a specific process; 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the. Heat Capacity Coefficients.
From www.numerade.com
SOLVED For many gases, the temperature dependence of the heat capacity Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Yaws, c.l., chemical properties handbook: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount. Heat Capacity Coefficients.
From byjus.com
The SI unit of specific heat capacity of a substance is Heat Capacity Coefficients Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Yaws, c.l., chemical properties handbook: Define heat capacity of an ideal gas for a specific process; Explain the difference between the heat. It is therefore an extensive property —its value is proportional to. Calculate the specific heat of an ideal gas for either. Heat Capacity Coefficients.
From www.cheresources.com
Estimating Heat Capacities for Solutions with Dissolved Solids Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. It is therefore an extensive property —its value is proportional to. Define heat capacity of an ideal gas for a specific process; In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount. Heat Capacity Coefficients.
From www.nagwa.com
Question Video Rearranging an Equation to Set Specific Heat Capacity Heat Capacity Coefficients Define heat capacity of an ideal gas for a specific process; Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. These occur at temperatures where the substance undergoes phase. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. 55 rows the table of specific. Heat Capacity Coefficients.
From www.researchgate.net
Heat capacity of pure water as function of temperature. Line calculated Heat Capacity Coefficients It is therefore an extensive property —its value is proportional to. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Heat capacity is determined by both the type. Heat Capacity Coefficients.
From forum.ansys.com
Heat transfer coefficient Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Heat capacity is determined by both the type. Heat Capacity Coefficients.
From www.slideshare.net
Heat capacity heatofformation Heat Capacity Coefficients Explain the difference between the heat. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; These occur at temperatures where the substance undergoes phase. Yaws, c.l., chemical properties handbook: Define heat capacity of an ideal gas for a specific process; Heat capacity is determined by both the type and amount of substance that absorbs. Heat Capacity Coefficients.
From www.coursehero.com
Methane (assumed to be in its idealgas state) is compressed Heat Capacity Coefficients Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Yaws, c.l., chemical properties handbook: The heat capacity is a smooth, continuous function of temperature except for a small. Heat Capacity Coefficients.
From www.chegg.com
Table 7.2.3 Heat Capacity Coefficients for the Heat Capacity Coefficients The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. Define heat capacity of an ideal gas for a specific process; Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well. Heat Capacity Coefficients.
From www.researchgate.net
4 th order Polynomial Parameters for the Specific Heat of Metal Oxides Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; The heat capacity is a smooth, continuous function of temperature except for a small number of discontinuities. These occur at temperatures where the substance undergoes phase. Yaws, c.l., chemical properties handbook: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount. Heat Capacity Coefficients.
From www.chegg.com
Solved Table B.2 Heat Capacities 1 1 1 1 1 Form l Heat Capacity Coefficients 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Explain the difference between the heat. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. Heat Capacity Coefficients.
From ar.inspiredpencil.com
Heat Capacity Chart Heat Capacity Coefficients Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Yaws, c.l., chemical properties handbook: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is determined by both the type and amount of substance that absorbs or releases. Heat Capacity Coefficients.