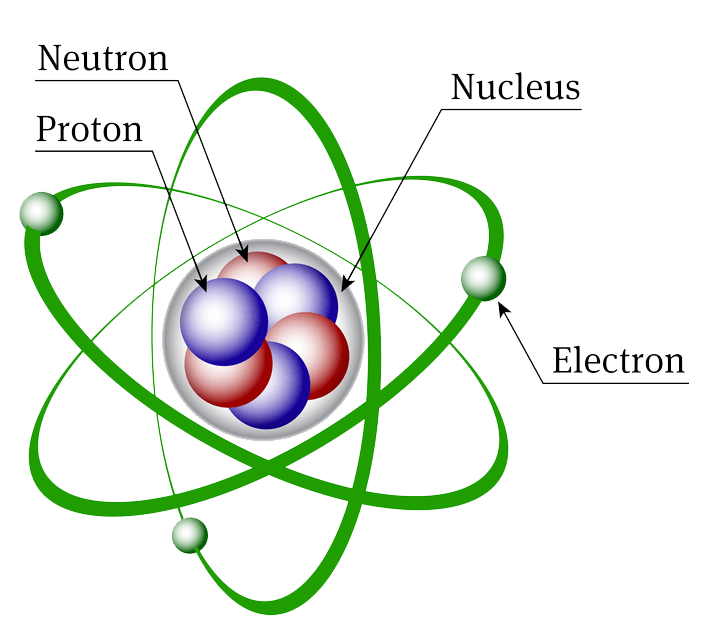
What Repels Electrons . An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Use conservation of charge to calculate quantities of charge transferred between objects. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Describe positive and negative electric charges. Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration.
from modernbiochemistry.weebly.com
Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Use conservation of charge to calculate quantities of charge transferred between objects. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Describe positive and negative electric charges.
Atomic Structure Biochemistry
What Repels Electrons Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Describe positive and negative electric charges. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Use conservation of charge to calculate quantities of charge transferred between objects. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron;
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Repels Electrons Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. Use conservation of charge to calculate quantities of charge transferred between objects. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Quantum mechanics tells us that it costs a. What Repels Electrons.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Repels Electrons Describe positive and negative electric charges. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Use conservation of charge to calculate quantities of charge transferred between objects. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have. What Repels Electrons.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry I What Repels Electrons Use conservation of charge to calculate quantities of charge transferred between objects. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. Describe positive and negative. What Repels Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Repels Electrons According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Describe positive and negative electric charges. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. A revolving electron would transform the atom into a miniature radio station,. What Repels Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. An electron has. What Repels Electrons.
From www.wonkeedonkeetools.co.uk
How does a work? What Repels Electrons A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Use conservation of charge to calculate quantities of charge transferred between objects. Atoms that. What Repels Electrons.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID205768 What Repels Electrons Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force. What Repels Electrons.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Repels Electrons Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Use conservation of charge to calculate quantities of charge transferred between objects. According to classical mechanics, the electron. What Repels Electrons.
From byjus.com
The path around the nucleus in which electrons revolve is called orbits. What Repels Electrons An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electron affinity is a measure of the energy. What Repels Electrons.
From www.lifeschemistrypress.com
5. The reality of electrons Life's Chemistry Press What Repels Electrons Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the. What Repels Electrons.
From www.vecteezy.com
Similar Poles of Repel Each Other. Field Lines What Repels Electrons According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a. What Repels Electrons.
From www.breakingatom.com
Electron Affinity of The Elements What Repels Electrons According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Describe positive and negative electric charges. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; Use conservation of charge to calculate quantities of. What Repels Electrons.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology What Repels Electrons An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Electrons in the outermost energy levels of atoms can be shared or. What Repels Electrons.
From mmerevise.co.uk
Fundamental Particles and Electronic Configuration MME What Repels Electrons Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are. What Repels Electrons.
From ee-lab.blogspot.com
Bohr model of silicon atom Electronics And Engineering Lab What Repels Electrons Use conservation of charge to calculate quantities of charge transferred between objects. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. A revolving electron would transform the atom into a miniature radio station, the energy. What Repels Electrons.
From modernbiochemistry.weebly.com
Atomic Structure Biochemistry What Repels Electrons Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. An electron has the opposite charge properties to a positive charge, and a free electron will. What Repels Electrons.
From www.expii.com
Electrons — Structure & Properties Expii What Repels Electrons In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Describe positive and negative electric charges. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. An electron has the opposite charge properties to a positive charge, and a free. What Repels Electrons.
From stock.adobe.com
Strong force in the nucleus of an atom . This science diagram shows the What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Describe positive and negative electric charges. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps. What Repels Electrons.
From scienceinfo.com
What are Valence Electrons? What Repels Electrons Describe positive and negative electric charges. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Electron affinity is a measure of the energy released when an electron is added to an atom to. What Repels Electrons.
From mmerevise.co.uk
Atoms Questions and Revision MME What Repels Electrons Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Electrons. What Repels Electrons.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost What Repels Electrons Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Quantum mechanics tells us that it costs a lot of energy to localize an electron in. What Repels Electrons.
From sites.google.com
Unit 4 Lewis Dot Structures & Bonding Mrosla Science What Repels Electrons Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Quantum mechanics tells. What Repels Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Repels Electrons Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Describe positive and negative electric charges. According to classical mechanics, the electron would simply spiral into. What Repels Electrons.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. Use conservation of charge to calculate quantities of charge transferred between objects. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Quantum mechanics tells us that it costs a. What Repels Electrons.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2100315 What Repels Electrons Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a. What Repels Electrons.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction. What Repels Electrons.
From www.worksheetsplanet.com
What Is An Electron What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Describe positive and negative electric charges. Electrons can be removed from some objects using friction, simply by rubbing one substance. What Repels Electrons.
From www.scienceabc.com
What Are Valence Electrons and How To Find Them? Where Are They Located? What Repels Electrons Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes. What Repels Electrons.
From www.shutterstock.com
Simple Model Atom Structure Electrons Orbiting Stock Vector (Royalty What Repels Electrons Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; Use conservation of charge to calculate quantities of. What Repels Electrons.
From blog.praxilabs.com
Your Step by Step Guide to Find Valence Electrons praxilabs What Repels Electrons Use conservation of charge to calculate quantities of charge transferred between objects. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. According to classical mechanics,. What Repels Electrons.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements What Repels Electrons Use conservation of charge to calculate quantities of charge transferred between objects. An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. A revolving electron would transform the. What Repels Electrons.
From instrumentationtools.com
Difference between Electron and Proton What Repels Electrons An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. Atoms that have lost electrons and become positively charged are called positive ions, and atoms that have gained electrons and become negatively charged are called negative ions. Electrons can be removed from some objects using. What Repels Electrons.
From www.researchgate.net
Simplified illustration of the potential drop in the wall sheath [24 What Repels Electrons A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force. What Repels Electrons.
From www.aquaportail.com
Valence définition illustrée et explications What Repels Electrons Electrons in the outermost energy levels of atoms can be shared or transferred to achieve a stable electron configuration. Electron affinity is a measure of the energy released when an electron is added to an atom to create a negatively charged ion. According to classical mechanics, the electron would simply spiral into the nucleus and the atom would collapse. Quantum. What Repels Electrons.
From www.allthescience.org
What is an Electron? (with pictures) What Repels Electrons Electrons can be removed from some objects using friction, simply by rubbing one substance against another substance. In addition to keeping drawings stuck to the refrigerator, the electromagnetic force also keeps electrons in orbit. Quantum mechanics tells us that it costs a lot of energy to localize an electron in a small volume. Describe positive and negative electric charges. Atoms. What Repels Electrons.