Gas Diffusion Rates . Find out how to apply graham's. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. the diffusion rate depends on several factors: gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. The mass of the atoms or molecules; learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings.
from diffuserjaika.blogspot.com
the diffusion rate depends on several factors: gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. Find out how to apply graham's. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. The mass of the atoms or molecules;
Absolute Rate Of Diffusion Of Gas
Gas Diffusion Rates learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. the diffusion rate depends on several factors: Find out how to apply graham's. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. The mass of the atoms or molecules;
From medcell.org
Histology Of The Respiratory System Lab Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. Find out how to apply graham's. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how gaseous molecules disperse and escape through small openings, and. Gas Diffusion Rates.
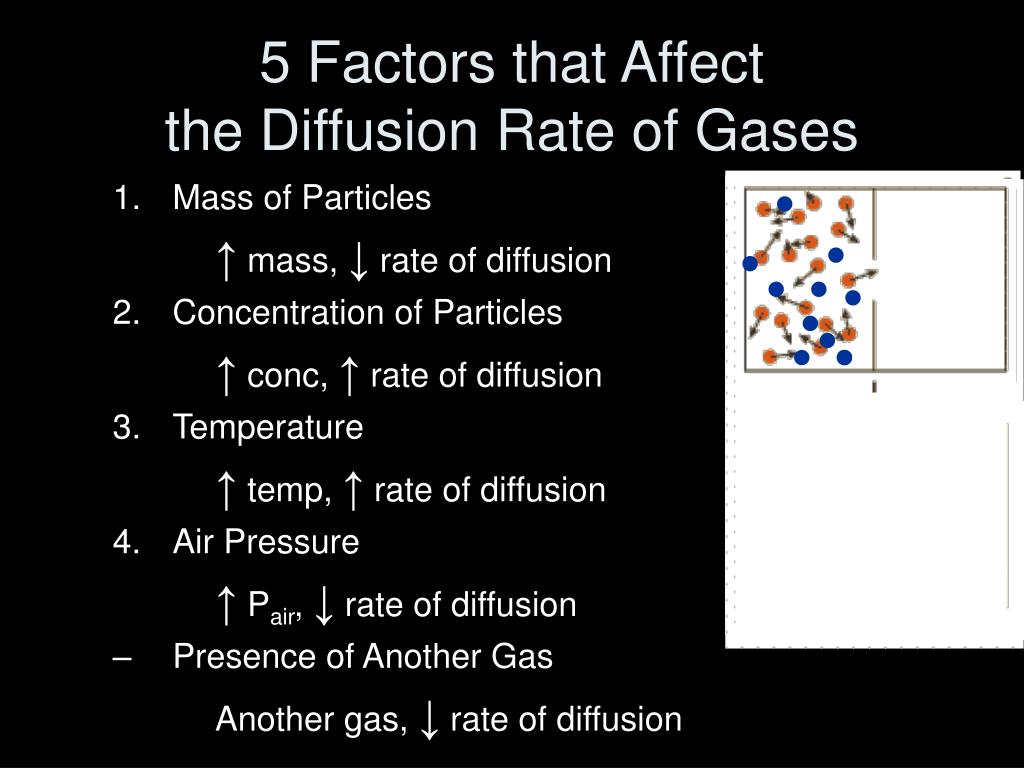
From www.slideserve.com
PPT General Properties of Gases PowerPoint Presentation, free Gas Diffusion Rates the diffusion rate depends on several factors: learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. Find out how to apply graham's. The mass of the atoms or molecules; gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how. Gas Diffusion Rates.
From www.slideserve.com
PPT Stoichiometry and Gases PowerPoint Presentation, free download Gas Diffusion Rates gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how the. Gas Diffusion Rates.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 Gas Diffusion Rates gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. the diffusion rate depends on several factors: learn the difference between effusion and diffusion of. Gas Diffusion Rates.
From www.researchgate.net
e Diffusion rate (a) and selectivity (b) of gases (H 2 , CO and CH 4 Gas Diffusion Rates gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. the diffusion rate depends on several factors: learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in. Gas Diffusion Rates.
From www.slideserve.com
PPT CHE 333 CLASS 12 PowerPoint Presentation, free download ID635177 Gas Diffusion Rates gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. Find out how to apply graham's. The mass of the atoms or molecules; the diffusion rate depends on several factors: learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass,. Gas Diffusion Rates.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 Gas Diffusion Rates learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn the difference between effusion and diffusion of gases, and how to apply graham's law to. Gas Diffusion Rates.
From www.slideserve.com
PPT Chpt 5 Gases PowerPoint Presentation, free download ID5503557 Gas Diffusion Rates the diffusion rate depends on several factors: The mass of the atoms or molecules; learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. Find out how. Gas Diffusion Rates.
From diffuserjaika.blogspot.com
Absolute Rate Of Diffusion Of Gas Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. the diffusion rate depends on several factors: learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how gaseous molecules disperse and escape through small openings, and how. Gas Diffusion Rates.
From www.researchgate.net
Diffusion rate (a) and selectivity (b) for noble gases (He, Ne and Ar Gas Diffusion Rates the diffusion rate depends on several factors: learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. The mass of the atoms or molecules; learn. Gas Diffusion Rates.
From www.researchgate.net
Diffusion rate (a) and selectivity (b) for noble gases (He, Ne and Ar Gas Diffusion Rates the diffusion rate depends on several factors: learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. Find out how to apply graham's. gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how. Gas Diffusion Rates.
From www.researchgate.net
(a) The diffusion rates and (b) the selectivity for gases to pass Gas Diffusion Rates Find out how to apply graham's. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. The mass of the atoms or molecules; learn how gaseous molecules disperse and escape through small openings, and. Gas Diffusion Rates.
From www.youtube.com
The rate of diffusion of two gases X and Y is in the ration of `15 Gas Diffusion Rates gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. Find out how to apply graham's. The mass of the atoms or molecules; learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how the rate of effusion or diffusion of a. Gas Diffusion Rates.
From chem.libretexts.org
5.9 Mean Free Path, Diffusion, and Effusion of Gases Chemistry Gas Diffusion Rates the diffusion rate depends on several factors: learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn the difference between effusion and diffusion of gases, and how to apply graham's law. Gas Diffusion Rates.
From www.researchgate.net
Schematic of the movement of gas molecules a normal gas diffusion and Gas Diffusion Rates Find out how to apply graham's. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure. Gas Diffusion Rates.
From www.slideshare.net
Chapter 11.4 Effusion and Diffusion Gas Diffusion Rates Find out how to apply graham's. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response to. Gas Diffusion Rates.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID257643 Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. gas diffusion is the movement of gas molecules from a region of higher concentration to a region. Gas Diffusion Rates.
From www.slideserve.com
PPT The Respiratory System PowerPoint Presentation, free download Gas Diffusion Rates the diffusion rate depends on several factors: The mass of the atoms or molecules; gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. Find out how to apply graham's. learn how. Gas Diffusion Rates.
From www.slideserve.com
PPT External Gas Transport PowerPoint Presentation, free download Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn the difference between effusion and diffusion of gases, and how to apply graham's law to. Gas Diffusion Rates.
From slideplayer.com
Gas Transfer (Diffusion of O2 and CO2) ppt download Gas Diffusion Rates learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. The mass of the atoms. Gas Diffusion Rates.
From www.youtube.com
CHEMISTRY 101 Diffusion and effusion of gases YouTube Gas Diffusion Rates the diffusion rate depends on several factors: learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. Find out how to apply graham's. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. The mass of the atoms or molecules;. Gas Diffusion Rates.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4144868 Gas Diffusion Rates learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. The mass of the atoms or molecules; learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how the rate of effusion or diffusion of a gas is inversely proportional to the. Gas Diffusion Rates.
From askfilo.com
The ratio of rate of diffusion for two gases is 12 if their molecular ma.. Gas Diffusion Rates learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. The mass. Gas Diffusion Rates.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Gas Diffusion Rates The mass of the atoms or molecules; learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response. Gas Diffusion Rates.
From www.researchgate.net
Influence of gas phase diffusion on the effective uptake coefficients Gas Diffusion Rates learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. the diffusion rate depends on several factors: learn how gaseous molecules diffuse and effuse in response to concentration gradients. Gas Diffusion Rates.
From byjus.com
34. if the ratio of Rates of diffusion of two gases X and Y is 9 1 Gas Diffusion Rates the diffusion rate depends on several factors: learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in. Gas Diffusion Rates.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4187263 Gas Diffusion Rates Find out how to apply graham's. the diffusion rate depends on several factors: learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. The mass of the atoms or molecules; learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. learn how gaseous molecules disperse and. Gas Diffusion Rates.
From www.researchgate.net
Comparison of diffusion values with values obtained from curve equation Gas Diffusion Rates learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn. Gas Diffusion Rates.
From mavink.com
Diffusion Coefficient Chart Gas Diffusion Rates the diffusion rate depends on several factors: gas diffusion is the movement of gas molecules from a region of higher concentration to a region of lower. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. The mass of the atoms or molecules; learn the difference between. Gas Diffusion Rates.
From www.researchgate.net
Gas diffusion rate and conductivity test of GDL (sample 1 is GDL29BC Gas Diffusion Rates The mass of the atoms or molecules; learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response. Gas Diffusion Rates.
From derangedphysiology.com
Diffusion of gases through the alveolar membrane Deranged Physiology Gas Diffusion Rates learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. The mass of the atoms or molecules; Find out how to apply graham's. learn the difference between effusion and diffusion of gases, and. Gas Diffusion Rates.
From oneseds.blogspot.com
Gas Particle Diagram Onesed Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. Find out how to apply graham's. The mass of the atoms or molecules; learn how gaseous molecules diffuse and effuse in response to concentration gradients and small openings. the diffusion rate depends on several factors:. Gas Diffusion Rates.
From www.toppr.com
The rates of diffusion of two gases A and B are in the mass ratio 14 Gas Diffusion Rates learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. Find out how to apply graham's. learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. The mass of the atoms or molecules; the diffusion rate depends. Gas Diffusion Rates.
From mavink.com
Rate Of Diffusion Graph Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules disperse and escape through small openings, and how their rates depend on temperature, mass, and. gas diffusion is the movement of gas molecules from a region of higher concentration to a. Gas Diffusion Rates.
From www.youtube.com
Factors that Affect Diffusion Rate YouTube Gas Diffusion Rates learn how the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. learn how gaseous molecules diffuse and effuse in response to concentration gradients and pressure differences. learn the difference between effusion and diffusion of gases, and how to apply graham's law to calculate gas. the. Gas Diffusion Rates.