Chemistry Lab For Specific Heat . Define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have greatest. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. This lab will have two experiment parts. Water, for example, has a specific heat of 1.0 cal/(g c). This value is high in comparison with the specific heats for other materials, such as. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined.
from studylib.net
This value is high in comparison with the specific heats for other materials, such as. Deduce which substance will have greatest. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. This lab will have two experiment parts. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Water, for example, has a specific heat of 1.0 cal/(g c). In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in.
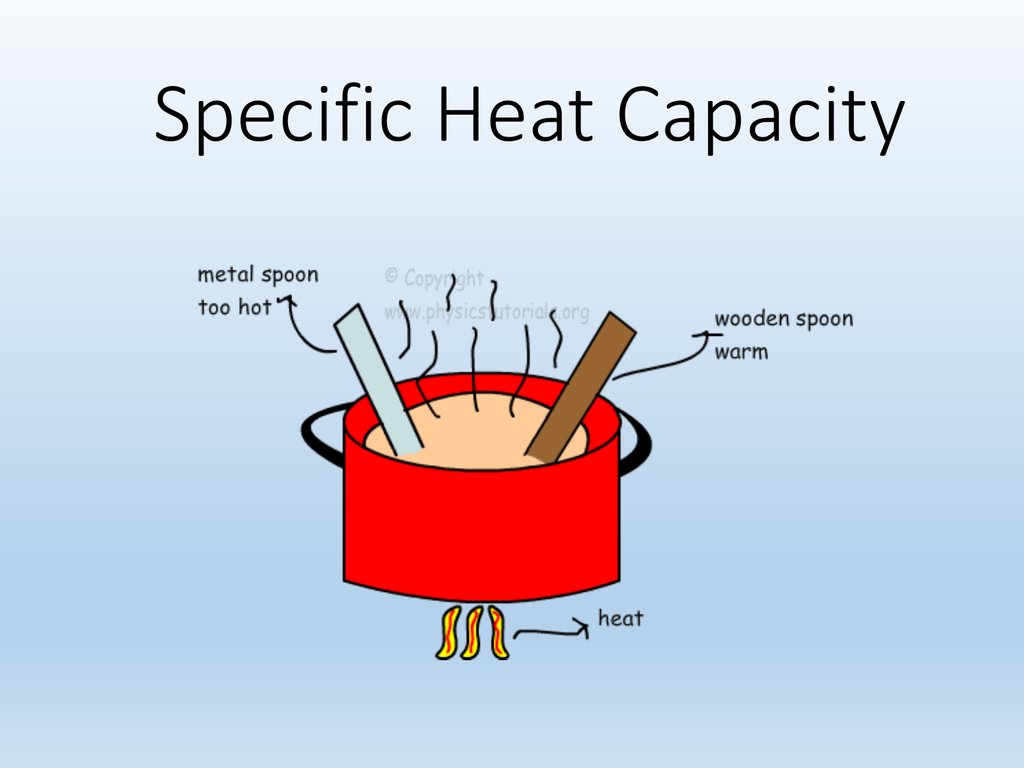
1.3 Specific Heat Capacity
Chemistry Lab For Specific Heat Water, for example, has a specific heat of 1.0 cal/(g c). The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Deduce which substance will have greatest. This value is high in comparison with the specific heats for other materials, such as. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. This lab will have two experiment parts. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Define heat capacity and specific heat capacity and differentiate between the two terms ; Water, for example, has a specific heat of 1.0 cal/(g c).
From www.studocu.com
1A Fall20 Online Lab Specific Heat Specific Heat Simulation Lab Chemistry Lab For Specific Heat The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. This lab will have two experiment parts. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Water, for example, has a specific heat of 1.0. Chemistry Lab For Specific Heat.
From www.chegg.com
Solved Experiment 25 Report Sheet Date A. Specific Heat of a Chemistry Lab For Specific Heat In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. This. Chemistry Lab For Specific Heat.
From ciderhousetech.com.au
Specific Heat Experiment Cider House Tech Chemistry Lab For Specific Heat Deduce which substance will have greatest. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. Define heat capacity and specific heat capacity and differentiate between the two terms ; The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a.. Chemistry Lab For Specific Heat.
From studylib.net
Chemistry 1 Unit 2 Calorimetry Lab Chemistry Lab For Specific Heat In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a. Chemistry Lab For Specific Heat.
From studylib.net
1.3 Specific Heat Capacity Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Define heat. Chemistry Lab For Specific Heat.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Chemistry Lab For Specific Heat To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. This value is high in comparison with the specific heats for other materials, such as. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. This lab will have. Chemistry Lab For Specific Heat.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. To get a more accurate measure for specific heat of the metal, the heat capacity of. Chemistry Lab For Specific Heat.
From www.youtube.com
Chemistry 10.2 Specific Heat Capacity YouTube Chemistry Lab For Specific Heat The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Water, for example, has a specific heat of 1.0 cal/(g c). To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The specific heat of. Chemistry Lab For Specific Heat.
From studylib.net
Experiment 15 Specific Heat of a Metal Chemistry Lab For Specific Heat To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Deduce which substance will have greatest. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. In this lab, students will get a general idea of. Chemistry Lab For Specific Heat.
From www.slideserve.com
PPT LAB Specific Heat of a Metal PowerPoint Presentation, free Chemistry Lab For Specific Heat Deduce which substance will have greatest. Water, for example, has a specific heat of 1.0 cal/(g c). Define heat capacity and specific heat capacity and differentiate between the two terms ; The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. To get a more accurate measure for specific heat. Chemistry Lab For Specific Heat.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. This lab will have two. Chemistry Lab For Specific Heat.
From www.youtube.com
Specific heat lab YouTube Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Water, for example, has a specific heat of 1.0 cal/(g c). To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Define heat capacity and specific heat capacity and. Chemistry Lab For Specific Heat.
From www.youtube.com
Chemistry Dearmond Virtual Lab Specific Heat Part 1 YouTube Chemistry Lab For Specific Heat The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. This lab will have two experiment parts. This value is high in comparison with the specific heats for other materials, such as. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different. Chemistry Lab For Specific Heat.
From www.chegg.com
Solved Lab 2 Specific Heat In this lab we will take samples Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change. Chemistry Lab For Specific Heat.
From studylib.net
LAB Specific Heat of a Metal Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Water, for example, has a specific heat of 1.0 cal/(g c). The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. Define heat capacity and specific heat capacity and differentiate between the two. Chemistry Lab For Specific Heat.
From www.youtube.com
Chemistry Unit 9 Lecture 11 PostLab Specific Heat Capacity YouTube Chemistry Lab For Specific Heat This lab will have two experiment parts. Define heat capacity and specific heat capacity and differentiate between the two terms ; In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific. Chemistry Lab For Specific Heat.
From www.youtube.com
Identifying an Unknown Metal Based on the Specific Heat and Density Lab Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. Deduce which substance will have. Chemistry Lab For Specific Heat.
From studylib.net
Lab 33 Specific Heat Chemistry Lab For Specific Heat The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. The specific heat of a substance can be used to calculate the temperature change that a. Chemistry Lab For Specific Heat.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Chemistry Lab For Specific Heat The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c.. Chemistry Lab For Specific Heat.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Chemistry Lab For Specific Heat The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be. Chemistry Lab For Specific Heat.
From kimora-kbowen.blogspot.com
Temperature and Specific Heat Lab 4 Answers Chemistry Lab For Specific Heat The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. In this lab, students will get a general idea of specific heat by investigating the mixing of two liquids at different temperatures. Define heat capacity and specific heat capacity and differentiate between the two terms ; The. Chemistry Lab For Specific Heat.
From studylib.net
chemistry lab specific heat of a metal Chemistry Lab For Specific Heat Water, for example, has a specific heat of 1.0 cal/(g c). Define heat capacity and specific heat capacity and differentiate between the two terms ; The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. The first is to determine the heat capacity of a calorimeter in a constant pressure. Chemistry Lab For Specific Heat.
From studylib.net
LAB FOUR Specific Heat of a Metal Objective Introduction Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Define heat capacity and specific heat capacity and differentiate between the two terms ; The specific heat of a. Chemistry Lab For Specific Heat.
From www.chegg.com
Solved Lab 1 Specific Heat Objective After completing this Chemistry Lab For Specific Heat The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Deduce which substance will have greatest. The first is to determine the heat. Chemistry Lab For Specific Heat.
From www.youtube.com
Chapter 1 Heat [ Finding Specific Heat of a Solid ] (Experiment) AP Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Deduce which substance will have greatest. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The. Chemistry Lab For Specific Heat.
From www.chegg.com
Solved EXPERIMENT 1 DETERMINATION OF SPECIFIC HEAT OF A Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. This value is high in comparison with the specific heats for other materials, such as. Water, for example, has a specific heat of 1.0 cal/(g c). The specific heat of a substance can be used to calculate the temperature change. Chemistry Lab For Specific Heat.
From www.studocu.com
Lab2specificheat 02 14 09 Chemistry 108 lab Name Chemistry Lab For Specific Heat The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. This lab will have two experiment parts. This value is high in comparison with the specific heats for other materials, such as.. Chemistry Lab For Specific Heat.
From www.studocu.com
Of Lab 11 Specific Heat Andria Swortzsh PHY 101 Name LAB Specific Chemistry Lab For Specific Heat The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. This value is high in comparison with the specific heats for other materials, such as. In this lab, students will get a. Chemistry Lab For Specific Heat.
From studylib.net
Calorimetry Lab Specific Heat Capacity Chemistry Lab For Specific Heat Water, for example, has a specific heat of 1.0 cal/(g c). Deduce which substance will have greatest. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. The specific heat of a. Chemistry Lab For Specific Heat.
From heatmozac.blogspot.com
CHAPTER 4 HEAT 4.2 Specific Heat Capacity Experiments Chemistry Lab For Specific Heat This value is high in comparison with the specific heats for other materials, such as. To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. Water, for example, has a specific heat of 1.0 cal/(g c). Define heat capacity and specific heat capacity and differentiate between the two terms. Chemistry Lab For Specific Heat.
From www.thoughtco.com
Specific Heat Capacity Definition Chemistry Lab For Specific Heat The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. In this lab, students will get a general idea of specific heat by investigating the mixing of. Chemistry Lab For Specific Heat.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Chemistry Lab For Specific Heat To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00°c. Define heat capacity and specific heat capacity and differentiate between the two terms ; In this lab, students will. Chemistry Lab For Specific Heat.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube Chemistry Lab For Specific Heat The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. This lab will have two experiment parts. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. Define heat capacity and specific heat. Chemistry Lab For Specific Heat.
From www.slideserve.com
PPT LAB Specific Heat of a Metal PowerPoint Presentation, free Chemistry Lab For Specific Heat The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. Deduce which substance will have greatest. The first is to determine the heat capacity of a calorimeter in a constant pressure environment (a. Water, for example, has a specific heat of 1.0 cal/(g c). In this lab,. Chemistry Lab For Specific Heat.
From www.coursehero.com
[Solved] Lab 6 Specific Heats and Calorimetry General Chemistry I Chemistry Lab For Specific Heat To get a more accurate measure for specific heat of the metal, the heat capacity of the calorimeter can be determined. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. Define heat capacity and specific heat capacity and differentiate between the two terms ; In this. Chemistry Lab For Specific Heat.