Magnesium Sulfate Density . Densities of aqueous solutions of inorganic sodium salts. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Density of inorganic sodium salts in water is plotted as. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). It is highly soluble in water and has various uses in. Magnesium sulfate is a white crystalline substance with the formula mgso4. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Changes in density of aqueous solutions with changes in concentration at 20°c. The structure of a magnesium sulfate molecule is illustrated below. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation.
from material-properties.org
Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Changes in density of aqueous solutions with changes in concentration at 20°c. Magnesium sulfate is a white crystalline substance with the formula mgso4. The structure of a magnesium sulfate molecule is illustrated below. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). It is highly soluble in water and has various uses in. Density of inorganic sodium salts in water is plotted as. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Densities of aqueous solutions of inorganic sodium salts.
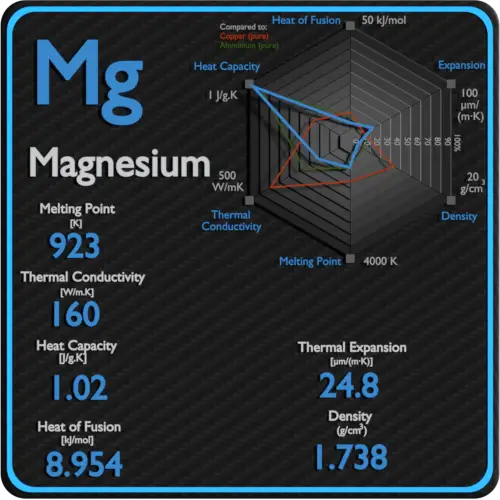
Magnesium Properties Price Applications Production
Magnesium Sulfate Density Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Changes in density of aqueous solutions with changes in concentration at 20°c. Magnesium sulfate is a white crystalline substance with the formula mgso4. The structure of a magnesium sulfate molecule is illustrated below. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. It is highly soluble in water and has various uses in. Densities of aqueous solutions of inorganic sodium salts. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Density of inorganic sodium salts in water is plotted as.
From www.bio-world.com
Magnesium Sulfate Anhydrous (7487889) bioWORLD Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Magnesium sulfate is a white crystalline substance with the formula mgso4. It is highly soluble in water and has various uses in. The structure of a magnesium sulfate molecule is illustrated below. Calculate the density or concentration of magnesium sulfate solutions. Magnesium Sulfate Density.
From obp.uest.gr
MgSO4 (Magnesium sulfate) Magnesium Sulfate Density Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Densities of aqueous solutions of inorganic sodium salts. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Density of inorganic sodium salts in water is plotted as. It is highly soluble in water and has various uses in. Magnesium sulfate is a white crystalline. Magnesium Sulfate Density.
From studylib.net
1. Product Identification Magnesium Sulfate Density It is highly soluble in water and has various uses in. Density of inorganic sodium salts in water is plotted as. Changes in density of aqueous solutions with changes in concentration at 20°c. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). The structure of a magnesium sulfate molecule is illustrated below. Magnesium sulfate is a. Magnesium Sulfate Density.
From www.youtube.com
Magnesium sulfate Wikipedia audio article YouTube Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Changes in density of aqueous solutions with changes in concentration at 20°c. It is highly soluble in water and has various uses in. The structure of a magnesium sulfate molecule is illustrated below. Note that mgso 4 molecules contain one mg. Magnesium Sulfate Density.
From www.smartscience.co.th
Magnesium Sulfate, Anhydrous smartscience Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. The structure of a magnesium sulfate molecule is illustrated below. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. It is highly soluble in water and has various uses in. Decomposes at around 1124°c (2055°f), though this can vary with heating rate.. Magnesium Sulfate Density.
From www.scribd.com
Material Safety Data Sheet Magnesium Sulfate Anhydrous MSDS PDF Magnesium Sulfate Density Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Density of inorganic sodium salts in water is plotted as. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. It is. Magnesium Sulfate Density.
From www.nagwa.com
Question Video Calculating the Mass of the Magnesium Sulfate Analyte Magnesium Sulfate Density Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Magnesium sulfate is a white crystalline substance with the formula mgso4. Density of inorganic sodium salts in water is plotted as. Densities of aqueous solutions of inorganic sodium salts. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Changes in density. Magnesium Sulfate Density.
From chemistry.stackexchange.com
experimental chemistry Calculating the density of a magnesium Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. Density of inorganic sodium salts in water is plotted as. The structure of a magnesium sulfate molecule is illustrated below. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Changes in density of aqueous solutions with changes in concentration at 20°c. 20 rows magnesium sulfate anhydrous. Magnesium Sulfate Density.
From ar.inspiredpencil.com
Magnesium Sulfate Refractive Index Magnesium Sulfate Density Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Changes in density of aqueous solutions with changes in concentration at 20°c. Density of inorganic sodium salts in water is plotted as. It is highly soluble in water and has various uses in. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Magnesium sulfate. Magnesium Sulfate Density.
From www.kimyalab.net
Kimyalab Magnezyum Sülfat MSDS Belgesi Magnesium Sulfate Heptahydrate Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Changes in density of aqueous solutions with changes in concentration at 20°c. Densities of aqueous solutions of inorganic sodium salts. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Magnesium sulfate is a white crystalline substance with. Magnesium Sulfate Density.
From en.wikipedia.org
FileMagnesium sulfate.JPG Wikipedia Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Densities of aqueous solutions of inorganic sodium salts. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per. Magnesium Sulfate Density.
From ar.inspiredpencil.com
Magnesium Sulfate Structure Magnesium Sulfate Density It is highly soluble in water and has various uses in. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Changes in density of aqueous solutions with changes in concentration at 20°c. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Densities of aqueous solutions of inorganic sodium salts. Magnesium sulfate is a. Magnesium Sulfate Density.
From www.scribd.com
Magnesium Sulfate Msds PDF Biodegradation Water Magnesium Sulfate Density Density of inorganic sodium salts in water is plotted as. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Densities of aqueous solutions of inorganic sodium salts. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Note that mgso 4 molecules contain one mg 2+ cation (the. Magnesium Sulfate Density.
From www.engineeringtoolbox.com
Density of aqueous solutions of some substances Magnesium Sulfate Density Density of inorganic sodium salts in water is plotted as. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). The structure of a magnesium sulfate molecule is illustrated below. It is highly soluble in water and has various uses in. Magnesium sulfate is a white crystalline substance with the formula mgso4. Calculate the density or concentration. Magnesium Sulfate Density.
From biobasic-asia.com
Magnesium Sulfate, anhydrous Bio Basic Asia Pacific Pte Ltd Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. Densities of aqueous solutions of inorganic sodium salts. Density of inorganic sodium salts in water is plotted as. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Calculate the density or concentration of magnesium sulfate solutions in water at. Magnesium Sulfate Density.
From material-properties.org
Magnesium Properties Price Applications Production Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Changes in density of aqueous solutions with changes in concentration at 20°c. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. It is highly soluble in water and has various uses in. Densities of. Magnesium Sulfate Density.
From irchemineral.com
Magnesium sulfate properties IRCHEM.co Magnesium Sulfate Density Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Density of inorganic sodium salts in water is plotted as. The structure of a magnesium sulfate molecule is illustrated. Magnesium Sulfate Density.
From www.fishersci.es
Sulfato de magnesio, anhidro, 99.5 mín., Thermo Scientific Chemicals Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. It is highly soluble in water and has various uses in. Changes in density of aqueous solutions with changes in concentration at 20°c. Density of inorganic sodium salts in water is plotted as. The structure of a magnesium sulfate molecule is. Magnesium Sulfate Density.
From www.researchgate.net
Magnesium sulfate solubility at various temperatures. Download Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Density of inorganic sodium salts in water is plotted as. It is highly soluble in water and has various uses in. Changes in density of aqueous solutions with changes in. Magnesium Sulfate Density.
From www.mcguffmedical.com
Magnesium Sulfate 50, 500mg/mL, SDV, 10mL Vial McGuff Medical Products Magnesium Sulfate Density Densities of aqueous solutions of inorganic sodium salts. Changes in density of aqueous solutions with changes in concentration at 20°c. Magnesium sulfate is a white crystalline substance with the formula mgso4. The structure of a magnesium sulfate molecule is illustrated below. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). It is highly soluble in water. Magnesium Sulfate Density.
From studylib.net
Magnesium Sulfate 26 Aqueous Solution ANSI Magnesium Sulfate Density Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. The structure of a magnesium sulfate molecule is illustrated below. Densities of aqueous solutions of inorganic sodium salts. Density of inorganic sodium salts in water is plotted as. It is highly soluble. Magnesium Sulfate Density.
From pediaa.com
Difference Between Magnesium Chloride and Magnesium Sulfate Magnesium Sulfate Density Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Densities of aqueous solutions of inorganic sodium salts. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. It is highly soluble in water and has various uses in. Changes in density of aqueous solutions with changes in concentration at 20°c. Density. Magnesium Sulfate Density.
From www.youtube.com
Molar Mass / Molecular Weight of MgSO4 Magnesium sulfate YouTube Magnesium Sulfate Density Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Magnesium sulfate is a white crystalline substance with the formula mgso4. It is highly soluble in water and has various uses in. Densities of aqueous solutions of inorganic sodium salts. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). The. Magnesium Sulfate Density.
From www.researchgate.net
Overview of magnesium chemistry as a function of temperature and Magnesium Sulfate Density Densities of aqueous solutions of inorganic sodium salts. Changes in density of aqueous solutions with changes in concentration at 20°c. Magnesium sulfate is a white crystalline substance with the formula mgso4. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. It is highly soluble in water and has various uses. Magnesium Sulfate Density.
From www.indiamart.com
Magnesium Sulphate Granules at Rs 12/kg Magnesium Sulphate in Indore Magnesium Sulfate Density It is highly soluble in water and has various uses in. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Density of inorganic sodium salts in water is plotted as. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter. Magnesium Sulfate Density.
From www.slideserve.com
PPT Hydrates PowerPoint Presentation, free download ID2110313 Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Density of inorganic sodium salts in water is plotted as. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Densities of aqueous solutions of inorganic sodium salts. Note that mgso 4 molecules contain one. Magnesium Sulfate Density.
From mavink.com
Magnesium Sulfate Compatibility Chart Magnesium Sulfate Density Changes in density of aqueous solutions with changes in concentration at 20°c. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Density of inorganic sodium. Magnesium Sulfate Density.
From fivestarchemicals.com
Magnesium Sulfate Magnesium Sulfate Density The structure of a magnesium sulfate molecule is illustrated below. Magnesium sulfate is a white crystalline substance with the formula mgso4. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Densities of aqueous solutions of inorganic sodium salts. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium. Magnesium Sulfate Density.
From proper-cooking.info
Magnesium Sulfate Structure Magnesium Sulfate Density Magnesium sulfate is a white crystalline substance with the formula mgso4. It is highly soluble in water and has various uses in. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Density of inorganic sodium salts in water is plotted as.. Magnesium Sulfate Density.
From stock.adobe.com
Magnesium Sulfate Properties and Chemical Compound Structure Stock Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. The structure of a magnesium sulfate molecule is illustrated below. Magnesium sulfate is a white crystalline substance with the formula mgso4. Note that mgso 4. Magnesium Sulfate Density.
From studylibplimsoll.z21.web.core.windows.net
Magnesium Density Vs Aluminum Magnesium Sulfate Density Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Densities of aqueous solutions of inorganic sodium salts. It is highly soluble in water and has various uses in. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Density of inorganic sodium salts in water is plotted as. Note that mgso. Magnesium Sulfate Density.
From www.tradeindia.com
Crystal Form Dried Magnesium Sulphate Heptahydrate For Industrial Magnesium Sulfate Density 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Densities of aqueous solutions of inorganic sodium salts. It is highly soluble in water and has various uses in. Magnesium sulfate is a white crystalline substance with the formula mgso4. Note that mgso 4 molecules contain one mg 2+ cation (the. Magnesium Sulfate Density.
From www.researchgate.net
Specific gravity of sodium sulfate and magnesium sulfate [10 Magnesium Sulfate Density The structure of a magnesium sulfate molecule is illustrated below. Densities of aqueous solutions of inorganic sodium salts. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Note that mgso 4 molecules contain one mg 2+ cation (the magnesium ion). 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic. Magnesium Sulfate Density.
From www.dreamstime.com
Magnesium Sulfate, Salt, Molecular Structures, 3d Model, Structural Magnesium Sulfate Density The structure of a magnesium sulfate molecule is illustrated below. Decomposes at around 1124°c (2055°f), though this can vary with heating rate. Densities of aqueous solutions of inorganic sodium salts. 20 rows magnesium sulfate anhydrous weighs 2.66 gram per cubic centimeter or 2 660 kilogram per cubic meter, i.e. Magnesium sulfate is a white crystalline substance with the formula mgso4.. Magnesium Sulfate Density.
From www.mz-store.com
Which magnesium should you choose? The best forms of magnesium! Magnesium Sulfate Density Changes in density of aqueous solutions with changes in concentration at 20°c. The structure of a magnesium sulfate molecule is illustrated below. It is highly soluble in water and has various uses in. Calculate the density or concentration of magnesium sulfate solutions in water at different temperatures using interpolation. Decomposes at around 1124°c (2055°f), though this can vary with heating. Magnesium Sulfate Density.