Infrared Spectroscopy Chemistry . Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Absorbing groups in the infrared region absorb within a certain wavelength region. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques.
from www.frontiersin.org
Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Absorbing groups in the infrared region absorb within a certain wavelength region. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. This can be analyzed in three ways. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques.
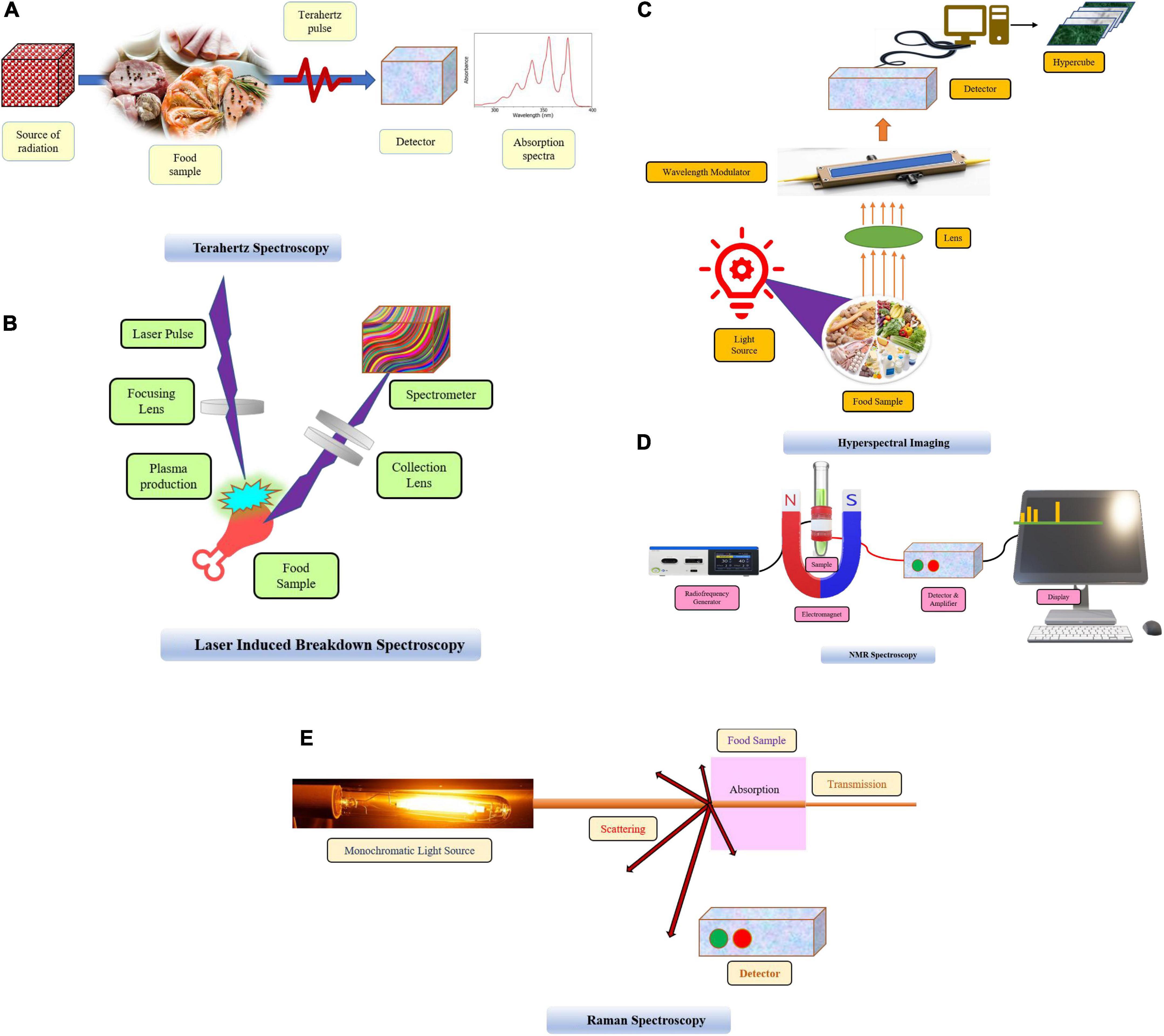
Frontiers Spectroscopic techniques for authentication of animal
Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Absorbing groups in the infrared region absorb within a certain wavelength region.
From awesomehome.co
Ir Spectroscopy Table Of Peaks Awesome Home Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding. Infrared Spectroscopy Chemistry.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Infrared Spectroscopy Chemistry Absorbing groups in the infrared region absorb within a certain wavelength region. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. An infrared. Infrared Spectroscopy Chemistry.
From www.youtube.com
Infrared spectroscopy AS Chemistry AQA New spec YouTube Infrared Spectroscopy Chemistry Absorbing groups in the infrared region absorb within a certain wavelength region. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies,. Infrared Spectroscopy Chemistry.
From www.youtube.com
IR Spectroscopy Basic Introduction YouTube Infrared Spectroscopy Chemistry The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Absorbing groups in the infrared region absorb within a certain wavelength region. The absorption peaks within this region are usually sharper when compared with absorption peaks from the. Infrared Spectroscopy Chemistry.
From chem.libretexts.org
4.4 Infrared spectroscopy Chemistry LibreTexts Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Infrared (ir) spectroscopy is one. Infrared Spectroscopy Chemistry.
From chemistry.stackexchange.com
organic chemistry Interpreting Infrared Spectroscopy (IR) Spectra Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. This can be analyzed in three ways. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Absorbing groups in the infrared region absorb within. Infrared Spectroscopy Chemistry.
From chem.libretexts.org
10.3 UV/Vis and IR Spectroscopy Chemistry LibreTexts Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Infrared spectroscopy is. Infrared Spectroscopy Chemistry.
From courses.lumenlearning.com
Infrared Spectroscopy MCC Organic Chemistry Infrared Spectroscopy Chemistry The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. This can be analyzed in three ways. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation.. Infrared Spectroscopy Chemistry.
From chem.libretexts.org
4.3 Infrared spectroscopy Chemistry LibreTexts Infrared Spectroscopy Chemistry The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Absorbing groups in. Infrared Spectroscopy Chemistry.
From www.studyorgo.com
IR Spectroscopy Review Infrared Spectroscopy Chemistry The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Infrared spectroscopy is the analysis of infrared light interacting with. Infrared Spectroscopy Chemistry.
From www.pinterest.co.kr
IR Spectroscopy Chart 1 Organic chemistry, Organic chemistry study Infrared Spectroscopy Chemistry Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The portion of the infrared region most useful for analysis of organic compounds. Infrared Spectroscopy Chemistry.
From encyclopedia.pub
NearInfrared Spectroscopy Encyclopedia MDPI Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. This can be analyzed in three ways. The number of possible vibrations for a molecule is determined. Infrared Spectroscopy Chemistry.
From ar.inspiredpencil.com
Ir Spectrum Table Aromatic Ring Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways. The portion of the infrared region. Infrared Spectroscopy Chemistry.
From www.iitianacademy.com
CIE AS Level Chemistry 9701 Topic 22 Analytical techniques Unit 22 Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. This can be analyzed in three ways. Absorbing groups in the infrared region absorb within a certain wavelength region. The absorption peaks within this region are usually sharper when compared. Infrared Spectroscopy Chemistry.
From www.frontiersin.org
Frontiers Spectroscopic techniques for authentication of animal Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013. Infrared Spectroscopy Chemistry.
From www.bionity.com
Infrared (IR) Spectroscopy Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Describe the vibrations of carbon. Infrared Spectroscopy Chemistry.
From www.alamy.com
Fourier Transform Infrared Spectroscopy (FTIR) spectrometer in Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. This can be. Infrared Spectroscopy Chemistry.
From www.slideserve.com
PPT Infrared Spectroscopy PowerPoint Presentation, free download ID Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. The absorption peaks within this region are usually sharper when compared with absorption. Infrared Spectroscopy Chemistry.
From chemistry.stackexchange.com
organic chemistry Interpreting Infrared Spectroscopy (IR) Spectra Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. The absorption peaks within this region are usually sharper when compared with absorption peaks from the. Infrared Spectroscopy Chemistry.
From ar.inspiredpencil.com
Ir Spectroscopy Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Absorbing groups in the infrared region absorb. Infrared Spectroscopy Chemistry.
From www.youtube.com
Infrared Spectroscopy YouTube Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The portion of the infrared region most useful for analysis of. Infrared Spectroscopy Chemistry.
From delloyd.50megs.com
Infrared and FTIR spectroscopy Instrument Infrared Spectroscopy Chemistry The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. This can be analyzed in three ways. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Infrared spectroscopy is. Infrared Spectroscopy Chemistry.
From brokeasshome.com
Infrared Spectroscopy Table Ocr Infrared Spectroscopy Chemistry Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. This can be analyzed in three ways. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Infrared (ir) spectroscopy. Infrared Spectroscopy Chemistry.
From www.youtube.com
Instrumentation of infrared spectroscopy (Visual demonstration Infrared Spectroscopy Chemistry The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The portion. Infrared Spectroscopy Chemistry.
From studylib.net
Infrared Spectroscopy Organic Chemistry with Joe Sloop Infrared Spectroscopy Chemistry Absorbing groups in the infrared region absorb within a certain wavelength region. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The portion of the infrared region most useful for analysis. Infrared Spectroscopy Chemistry.
From chemistry.com.pk
Free Download Infrared Spectroscopy By James M. Thompson Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. This can be analyzed. Infrared Spectroscopy Chemistry.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Infrared Spectroscopy Chemistry The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared (ir) spectroscopy is one of the. Infrared Spectroscopy Chemistry.
From www.youtube.com
Infrared Spectroscopy AQA ALevel Organic Chemistry YouTube Infrared Spectroscopy Chemistry Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. The number of possible vibrations for a molecule. Infrared Spectroscopy Chemistry.
From www.youtube.com
Organic Chemistry Infrared Spectroscopy YouTube Infrared Spectroscopy Chemistry An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring the absorptions. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Absorbing groups in the infrared region absorb within. Infrared Spectroscopy Chemistry.
From www.youtube.com
ASLevel Chemistry Infrared Spectroscopy Part 4 YouTube Infrared Spectroscopy Chemistry The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. This can be analyzed in three ways. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. Describe the vibrations. Infrared Spectroscopy Chemistry.
From en.wikipedia.org
Infrared spectroscopy Wikipedia Infrared Spectroscopy Chemistry The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. An infrared. Infrared Spectroscopy Chemistry.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent Infrared Spectroscopy Chemistry Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Absorbing groups in the infrared region absorb within a certain wavelength region. An infrared spectrometer analyses a compound by passing infrared radiation, over a range of different frequencies, through a sample and measuring. Infrared Spectroscopy Chemistry.
From organicchemistoncall.com
Most Commonly Used IR Spectroscopy Values In Organic Chemistry The Infrared Spectroscopy Chemistry Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. This can be analyzed in three ways. The absorption peaks within this region are usually sharper when compared with absorption peaks from the ultraviolet and visible regions. The number of possible vibrations for a molecule is determined by the degrees of freedom of the molecule. The. Infrared Spectroscopy Chemistry.
From www.tpsearchtool.com
Atr Fourier Transform Infrared Spectroscopy Ftir Spectra Of The Pan Images Infrared Spectroscopy Chemistry The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 hz. Absorbing groups in the infrared region absorb within a certain wavelength region. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared. Infrared Spectroscopy Chemistry.
From www.linstitute.net
CIE A Level Chemistry复习笔记4.1.1 InfraRed Spectroscopy翰林国际教育 Infrared Spectroscopy Chemistry Infrared (ir) spectroscopy is one of the most common and widely used spectroscopic techniques. Describe the vibrations of carbon dioxide (co 2) and determine which ones absorb infrared radiation. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500. Infrared Spectroscopy Chemistry.