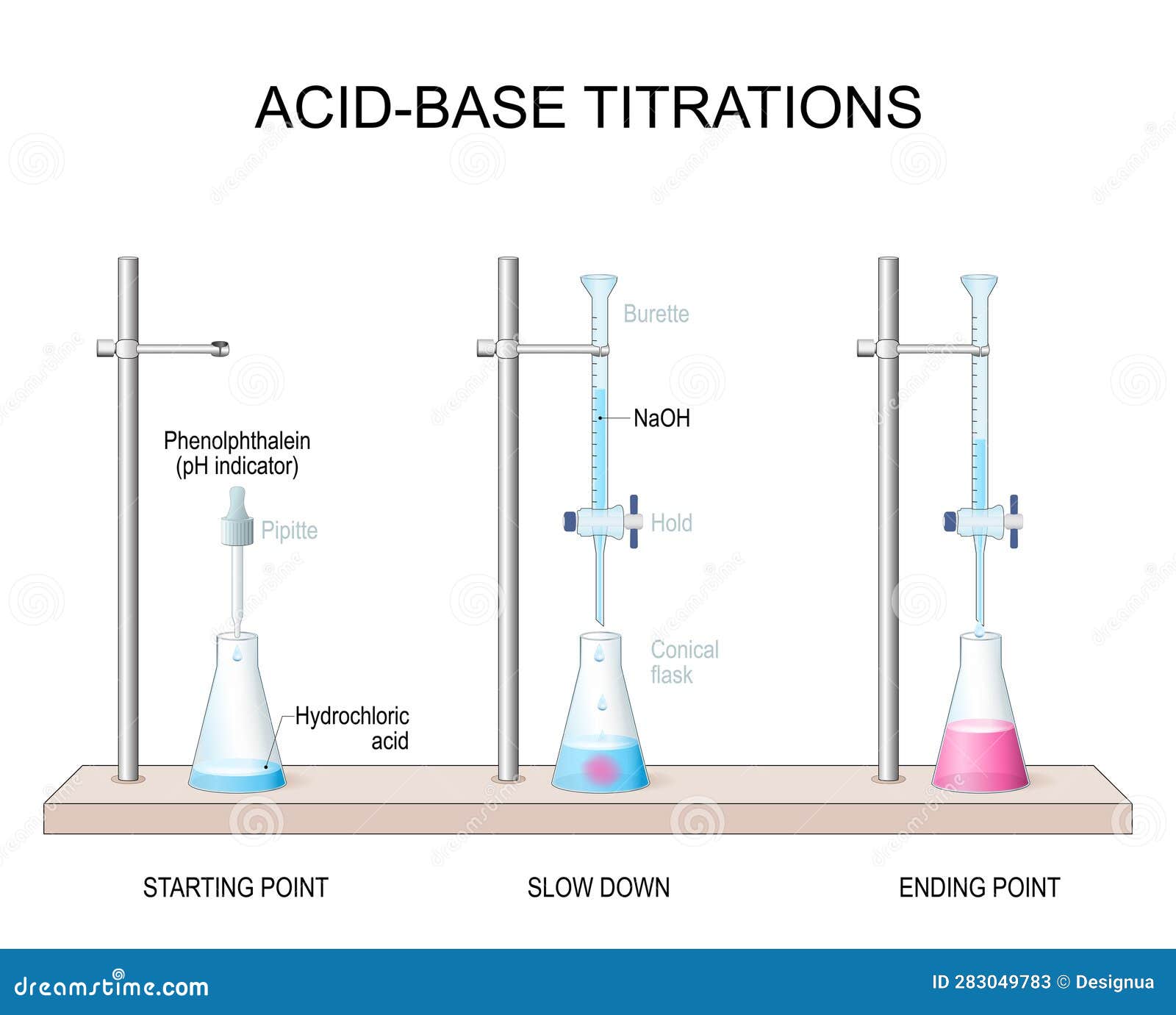
Titration What Is Measured . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. For example, you can use it to find the concentration. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the.
from ar.inspiredpencil.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. For example, you can use it to find the concentration. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
Titration Diagram
Titration What Is Measured Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of. Titration What Is Measured.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration What Is Measured.
From byjus.com
Why is titration used? Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. For example, you can use it to find the concentration. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is a laboratory technique. Titration What Is Measured.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration What Is Measured For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical technique that allows the quantitative determination. Titration What Is Measured.
From www.numerade.com
SOLVED Identify each type of titration curve Note that the analyte is Titration What Is Measured Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is a way of measuring the concentration of something, usually the concentration of. Titration What Is Measured.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration What Is Measured.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For example, you can use it to find the concentration. Titration is the process in which. Titration What Is Measured.
From www.numerade.com
SOLVED 2 What is the true regarding to the basic principle of Titration What Is Measured Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Titration What Is Measured.
From facts.net
8 Captivating Facts About AcidBase Titration Titration What Is Measured For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. A titration is a laboratory technique used to precisely measure molar concentration. Titration What Is Measured.
From ar.inspiredpencil.com
Titration Setup Diagram Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration What Is Measured.
From www.slideserve.com
PPT V. AcidBase Titration Titration is the process of adding a Titration What Is Measured Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in. Titration What Is Measured.
From slideplayer.com
Which items do you think are ACIDS & which are BASES? ppt download Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. For example, you can use it to find the concentration. Titration is an analytical. Titration What Is Measured.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample. Titration What Is Measured.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. For example, you can use it to find the concentration. Titration is the slow addition of. Titration What Is Measured.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration What Is Measured.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another. Titration What Is Measured.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration What Is Measured Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration What Is Measured.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration What Is Measured.
From www.chegg.com
Solved QUESTION Before Titration 0.4590 g KHP was measured Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the process in which one solution is added to another solution such that it. Titration What Is Measured.
From ar.inspiredpencil.com
Titration Diagram Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration What Is Measured.
From www.slideserve.com
PPT V. AcidBase Titration Titration is the process of adding a Titration What Is Measured Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is. Titration What Is Measured.
From pdfslide.net
(PPT) (8.4) AcidBase Titration. What is Titration? Demo Time! The Titration What Is Measured A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the. Titration What Is Measured.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration What Is Measured Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. For example, you can use it to find the concentration. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is the slow addition of one solution. Titration What Is Measured.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Titration What Is Measured A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual. Titration What Is Measured.
From www.youtube.com
Total Alkalinity Titration Method and Calculations YouTube Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration What Is Measured.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration What Is Measured Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. Titration What Is Measured.
From www.chegg.com
Solved Titration Hints To remove air bubbles let the titrant Titration What Is Measured For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is the process in which one solution. Titration What Is Measured.
From theedge.com.hk
Chemistry How To Titration The Edge Titration What Is Measured Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the process in which one solution is added to another solution such that it. Titration What Is Measured.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration What Is Measured Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration What Is Measured.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration What Is Measured Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is the process of determining the quantity of one substance by adding measured amounts of another. Titration What Is Measured.
From slideplayer.com
WarmUp Do not turn in pH practice! ppt download Titration What Is Measured Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a way of measuring the concentration of something, usually the concentration of. Titration What Is Measured.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration What Is Measured Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For example, you can use it to find the concentration. Titration is the process in which one solution. Titration What Is Measured.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration What Is Measured Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample. Titration What Is Measured.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration What Is Measured For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is an analytical technique that allows the quantitative determination of. Titration What Is Measured.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration What Is Measured A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity. Titration What Is Measured.