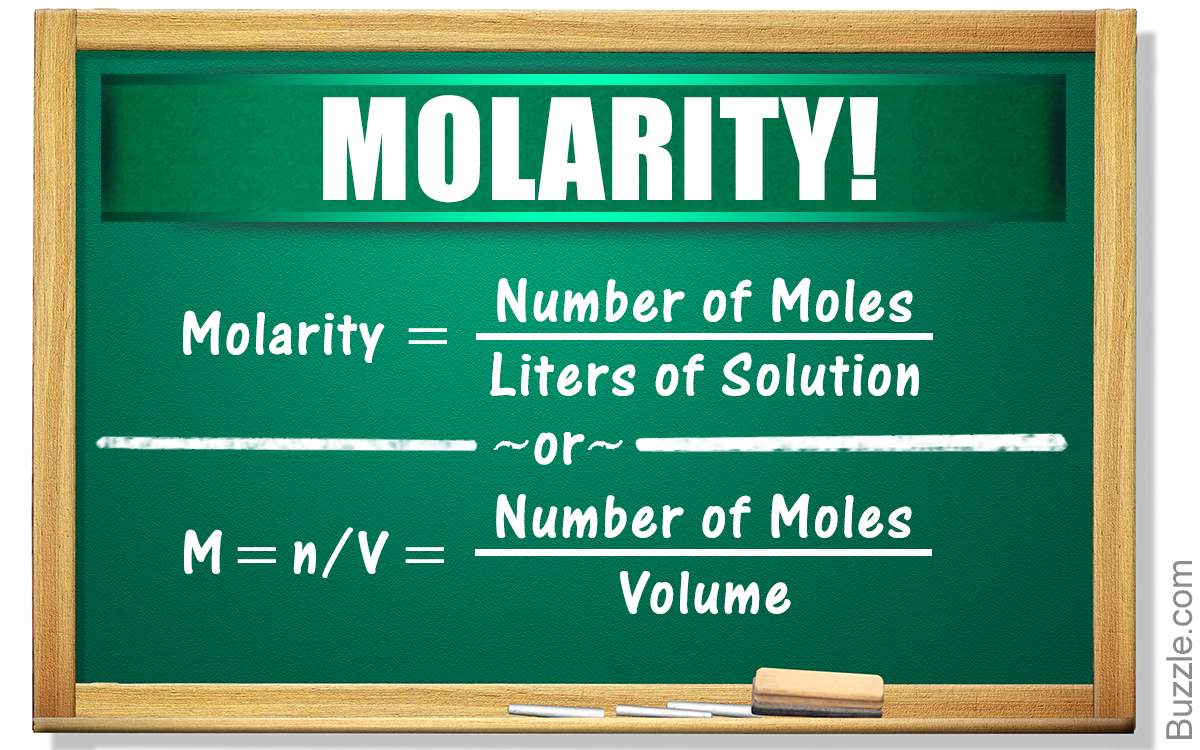
How To Calculate Osmolarity Concentration . Calculate the osmolarity of blood. [ #glucose# ] = 180 mg/100 ml; The concentrations of solutes are: An osmole (osmol) is 1 mol of particles that. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these. You multiply the molarity by the number of osmoles that each solute produces. [ #na^+# ] = 0.140 mol/l; This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic.
from ibiologia.com
You multiply the molarity by the number of osmoles that each solute produces. [ #na^+# ] = 0.140 mol/l; An osmole (osmol) is 1 mol of particles that. The concentrations of solutes are: [ #glucose# ] = 180 mg/100 ml; Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these.
How to calculate Osmolarity from Molarity?
How To Calculate Osmolarity Concentration You multiply the molarity by the number of osmoles that each solute produces. You multiply the molarity by the number of osmoles that each solute produces. The concentrations of solutes are: Calculate the osmolarity of blood. While any polar solvent could be used, these. [ #na^+# ] = 0.140 mol/l; This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. [ #glucose# ] = 180 mg/100 ml; An osmole (osmol) is 1 mol of particles that.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. [ #glucose# ] = 180 mg/100 ml; The concentrations of solutes are: While any polar solvent could be used, these. This article will explain how to calculate osmolarity using some example questions that. How To Calculate Osmolarity Concentration.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Calculate Osmolarity Concentration An osmole (osmol) is 1 mol of particles that. [ #na^+# ] = 0.140 mol/l; You multiply the molarity by the number of osmoles that each solute produces. The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these.. How To Calculate Osmolarity Concentration.
From fyordcqhv.blob.core.windows.net
How To Find Osmolality at Douglas Clyne blog How To Calculate Osmolarity Concentration Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. The concentrations of solutes are: An osmole (osmol) is 1 mol of particles that. This article will explain how to calculate osmolarity using some example questions that will. How To Calculate Osmolarity Concentration.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate Osmolarity Concentration [ #glucose# ] = 180 mg/100 ml; Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. While any polar solvent could be used, these. The concentrations of solutes are: [ #na^+# ] = 0.140 mol/l; You multiply the molarity by the number of osmoles. How To Calculate Osmolarity Concentration.
From ibiologia.com
How to calculate Osmolarity from Molarity? How To Calculate Osmolarity Concentration Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. The concentrations of solutes are: [ #glucose# ] = 180 mg/100 ml; This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. An osmole (osmol) is 1 mol of particles that.. How To Calculate Osmolarity Concentration.
From www.youtube.com
Calculated Osmolality YouTube How To Calculate Osmolarity Concentration You multiply the molarity by the number of osmoles that each solute produces. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. The concentrations of solutes are: An osmole (osmol) is 1 mol of particles that. Calculate the osmolarity of blood. While any polar solvent could be used, these.. How To Calculate Osmolarity Concentration.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate Osmolarity Concentration [ #na^+# ] = 0.140 mol/l; An osmole (osmol) is 1 mol of particles that. Calculate the osmolarity of blood. [ #glucose# ] = 180 mg/100 ml; The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. This article will explain how to calculate osmolarity using. How To Calculate Osmolarity Concentration.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate Osmolarity Concentration This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. While any polar solvent could be used, these. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Calculate the osmolarity of blood. You multiply the molarity by the number of. How To Calculate Osmolarity Concentration.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality How To Calculate Osmolarity Concentration [ #glucose# ] = 180 mg/100 ml; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. The. How To Calculate Osmolarity Concentration.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate Osmolarity Concentration While any polar solvent could be used, these. Calculate the osmolarity of blood. The concentrations of solutes are: [ #na^+# ] = 0.140 mol/l; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. [ #glucose# ] = 180 mg/100 ml; An osmole (osmol) is 1 mol of particles that. You. How To Calculate Osmolarity Concentration.
From www.youtube.com
Estimating Serum Osmolality using a Simple Formula YouTube How To Calculate Osmolarity Concentration This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. [ #na^+# ] = 0.140 mol/l; An osmole (osmol) is 1 mol of particles that. Calculate. How To Calculate Osmolarity Concentration.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Calculate Osmolarity Concentration [ #glucose# ] = 180 mg/100 ml; While any polar solvent could be used, these. An osmole (osmol) is 1 mol of particles that. You multiply the molarity by the number of osmoles that each solute produces. [ #na^+# ] = 0.140 mol/l; Calculate the osmolarity of blood. Osmolarity and osmolality are units of solute concentration that are often used. How To Calculate Osmolarity Concentration.
From www.studypool.com
SOLUTION 6 regulation of extracellular fluid osmolarity and sodium How To Calculate Osmolarity Concentration Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Calculate the osmolarity of blood. An osmole (osmol) is 1 mol of particles that. While any polar solvent could be used, these. [ #na^+# ] = 0.140 mol/l; The concentrations of solutes are: You multiply the molarity by the number of. How To Calculate Osmolarity Concentration.
From www.youtube.com
Review Molarity, percent concentration, and osmolarity YouTube How To Calculate Osmolarity Concentration Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. Calculate the osmolarity of blood. While any polar. How To Calculate Osmolarity Concentration.
From www.thetechedvocate.org
How to calculate osmolarity The Tech Edvocate How To Calculate Osmolarity Concentration This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. An osmole (osmol) is 1 mol of particles that. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. [ #glucose# ] = 180 mg/100 ml; [ #na^+# ] = 0.140. How To Calculate Osmolarity Concentration.
From classlibrarycordeiro.z21.web.core.windows.net
How To Calculate Molarity In Chemistry How To Calculate Osmolarity Concentration The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. Calculate the osmolarity of blood. While any polar solvent could be used, these. [ #na^+# ]. How To Calculate Osmolarity Concentration.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. While any polar solvent could be used, these. The concentrations of solutes are: An osmole (osmol) is 1 mol of particles that. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. [ #glucose# ] = 180 mg/100 ml; Osmolarity and osmolality are units of. How To Calculate Osmolarity Concentration.
From www.chegg.com
Solved Calculate the osmolarity of a 1.15 (m/v) KBr How To Calculate Osmolarity Concentration [ #na^+# ] = 0.140 mol/l; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. The concentrations of solutes are: Calculate the osmolarity of blood. An osmole (osmol) is 1 mol of particles that. This article will. How To Calculate Osmolarity Concentration.
From www.slideserve.com
PPT Intracellular vs. extracellular concentrations PowerPoint How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. [ #glucose# ] = 180 mg/100 ml; While any polar solvent could be. How To Calculate Osmolarity Concentration.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. You multiply the molarity by the number of osmoles that each solute produces. [ #glucose# ] = 180 mg/100 ml; While any polar solvent could be used, these. The concentrations of solutes are: An osmole (osmol) is 1 mol of particles that. This article will explain how to calculate osmolarity using some example questions that. How To Calculate Osmolarity Concentration.
From www.chegg.com
Solved Calculating Osmolarity Practice Worksheet 1. How To Calculate Osmolarity Concentration This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. An osmole (osmol) is 1 mol of particles that. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute. How To Calculate Osmolarity Concentration.
From droualb.faculty.mjc.edu
Chapter 4 How To Calculate Osmolarity Concentration While any polar solvent could be used, these. Calculate the osmolarity of blood. The concentrations of solutes are: This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. You multiply the molarity by the number of osmoles that each solute produces. [ #glucose# ] = 180 mg/100 ml; [ #na^+#. How To Calculate Osmolarity Concentration.
From www.slideserve.com
PPT Osmoregulation = keeping water and salt balanced in the body How To Calculate Osmolarity Concentration [ #glucose# ] = 180 mg/100 ml; An osmole (osmol) is 1 mol of particles that. The concentrations of solutes are: You multiply the molarity by the number of osmoles that each solute produces. [ #na^+# ] = 0.140 mol/l; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While. How To Calculate Osmolarity Concentration.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How To Calculate Osmolarity Concentration This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. The concentrations of solutes are: Calculate the osmolarity of blood. While any polar solvent could be used, these. [ #glucose# ] = 180 mg/100 ml; Osmolarity and osmolality are units of solute concentration that are often used in reference to. How To Calculate Osmolarity Concentration.
From hxebamyle.blob.core.windows.net
How To Calculate Osmolarity From Percent Solution at Carol Reyes blog How To Calculate Osmolarity Concentration An osmole (osmol) is 1 mol of particles that. You multiply the molarity by the number of osmoles that each solute produces. [ #glucose# ] = 180 mg/100 ml; This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. The concentrations of solutes are: Calculate the osmolarity of blood. [. How To Calculate Osmolarity Concentration.
From www.wikihow.com
How to Calculate Osmolarity Formulas, Examples, & More How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. [ #glucose# ] = 180 mg/100 ml; While any polar solvent could be used, these. [ #na^+# ] = 0.140 mol/l; An osmole (osmol) is 1 mol of particles that. The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You. How To Calculate Osmolarity Concentration.
From www.chegg.com
Solved Concentration Osmolarity . Ca 2.12.8 How To Calculate Osmolarity Concentration Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. While any polar solvent could be used, these. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of. How To Calculate Osmolarity Concentration.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki How To Calculate Osmolarity Concentration An osmole (osmol) is 1 mol of particles that. [ #glucose# ] = 180 mg/100 ml; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic.. How To Calculate Osmolarity Concentration.
From www.wikihow.com
How to Calculate Osmolarity Formulas, Examples, & More How To Calculate Osmolarity Concentration [ #na^+# ] = 0.140 mol/l; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. This article will explain how to calculate osmolarity using some example questions that. How To Calculate Osmolarity Concentration.
From www.youtube.com
Renal system 13 Osmolarity How to calculate osmotic pressure How To Calculate Osmolarity Concentration The concentrations of solutes are: You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. [ #glucose# ] = 180 mg/100 ml; This article will explain how to calculate. How To Calculate Osmolarity Concentration.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Calculate Osmolarity Concentration While any polar solvent could be used, these. You multiply the molarity by the number of osmoles that each solute produces. This article will explain how to calculate osmolarity using some example questions that will improve your understanding of the topic. An osmole (osmol) is 1 mol of particles that. Calculate the osmolarity of blood. The concentrations of solutes are:. How To Calculate Osmolarity Concentration.
From lemurianembassy.com
😍 Finding osmolarity. Calculate your own osmolarity. 20190125 How To Calculate Osmolarity Concentration [ #na^+# ] = 0.140 mol/l; Calculate the osmolarity of blood. An osmole (osmol) is 1 mol of particles that. The concentrations of solutes are: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. While any polar. How To Calculate Osmolarity Concentration.
From www.slideserve.com
PPT Chemical calculations used in medicine part 1 PowerPoint How To Calculate Osmolarity Concentration Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these. An osmole (osmol) is 1 mol of particles that. The concentrations of solutes are: [ #glucose# ] = 180 mg/100. How To Calculate Osmolarity Concentration.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry How To Calculate Osmolarity Concentration [ #glucose# ] = 180 mg/100 ml; You multiply the molarity by the number of osmoles that each solute produces. [ #na^+# ] = 0.140 mol/l; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Calculate the osmolarity of blood. This article will explain how to calculate osmolarity using some. How To Calculate Osmolarity Concentration.
From www.chegg.com
Solved Question 3 Calculate the osmolarity of the following How To Calculate Osmolarity Concentration The concentrations of solutes are: [ #glucose# ] = 180 mg/100 ml; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. While any polar solvent could be used,. How To Calculate Osmolarity Concentration.