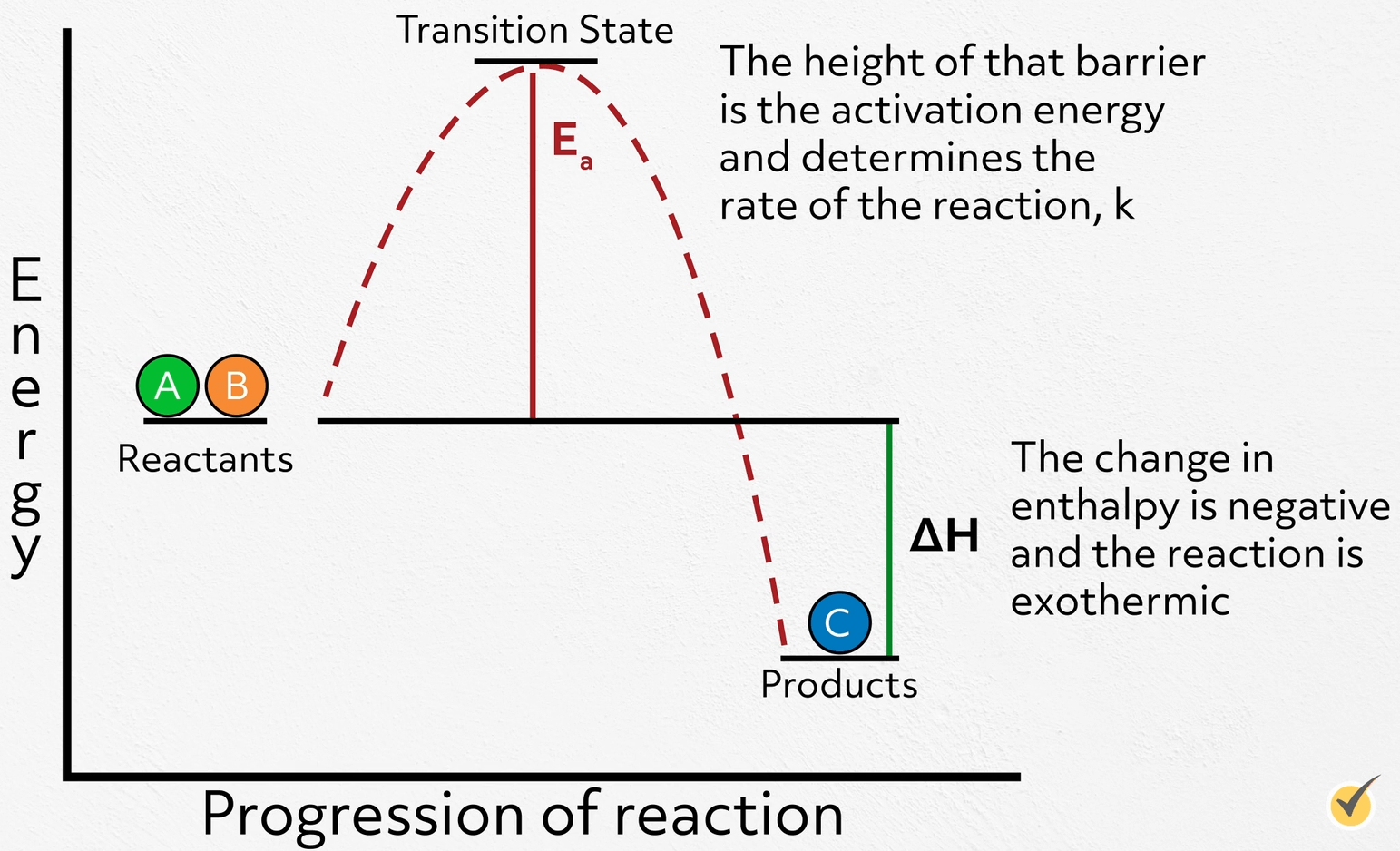
Explain How Activation Energy And Catalysts Affect Reaction Rates . this page describes and explains the way that adding a catalyst affects the rate of a reaction. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. the effect of a catalyst on the activation energy is shown on a chart called a. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Chart showing how the energy of. It assumes familiarity with basic concepts in the collision. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given.
from fyoefmgmv.blob.core.windows.net
Chart showing how the energy of. this page describes and explains the way that adding a catalyst affects the rate of a reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. It assumes familiarity with basic concepts in the collision. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. the effect of a catalyst on the activation energy is shown on a chart called a. this page explains how adding a catalyst affects the rate of a reaction.
Do Catalysts Increase Reaction Rate at Stephen Johnson blog
Explain How Activation Energy And Catalysts Affect Reaction Rates i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. the effect of a catalyst on the activation energy is shown on a chart called a. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. It assumes familiarity with basic concepts in the collision. Chart showing how the energy of. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself.
From dragonbeeotch4sschematic.z21.web.core.windows.net
Energy Diagram For Chemical Reaction Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes familiarity with basic concepts in the collision. this page describes and explains the way that adding a catalyst affects the rate of a reaction. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. revise how to measure rates of reaction. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Explain How Activation Energy And Catalysts Affect Reaction Rates this page explains how adding a catalyst affects the rate of a reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.nagwa.com
Question Video Determining How Catalysts Affect the Energy Changes Explain How Activation Energy And Catalysts Affect Reaction Rates i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. this page explains how adding a catalyst affects the rate of a reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From byjus.com
How does a catalyst increase the rate of a reaction? Explain How Activation Energy And Catalysts Affect Reaction Rates this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. the greater the frequency of successful collisions between reactant. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From fyoefmgmv.blob.core.windows.net
Do Catalysts Increase Reaction Rate at Stephen Johnson blog Explain How Activation Energy And Catalysts Affect Reaction Rates the effect of a catalyst on the activation energy is shown on a chart called a. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Explain How Activation Energy And Catalysts Affect Reaction Rates this page explains how adding a catalyst affects the rate of a reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. It assumes that you are already. i can describe what a catalyst does and explain how it affects. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From manuallistgranitise.z1.web.core.windows.net
Labeled Energy Diagram Chemistry Explain How Activation Energy And Catalysts Affect Reaction Rates Chart showing how the energy of. this page describes and explains the way that adding a catalyst affects the rate of a reaction. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. the effect of a catalyst on the activation energy is. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Explain How Activation Energy And Catalysts Affect Reaction Rates revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. i can describe what a catalyst does and explain how it affects the rate of reaction in. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Explain How Activation Energy And Catalysts Affect Reaction Rates a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. the effect of a catalyst on the activation energy is shown on a chart called a. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy,. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 Explain How Activation Energy And Catalysts Affect Reaction Rates Chart showing how the energy of. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. the effect of a catalyst on the activation energy is shown on a chart called a. It assumes familiarity with basic concepts in the collision. It assumes that. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Explain How Activation Energy And Catalysts Affect Reaction Rates revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. i can describe what a catalyst does and explain how it. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Explain How Activation Energy And Catalysts Affect Reaction Rates the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. It assumes that you are already. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. revise how to measure rates of reaction and how to impact the rate by. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Explain How Activation Energy And Catalysts Affect Reaction Rates Chart showing how the energy of. this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. It assumes that you are already. as can be seen from the arrhenius equation, the magnitude of the. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Explain How Activation Energy And Catalysts Affect Reaction Rates revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. the effect of a catalyst on the activation energy is shown on a chart called a. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. a catalyst is a substance. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.edukate.me
Explain The Effect of Catalyst on the Rate of Reaction A catalyst Explain How Activation Energy And Catalysts Affect Reaction Rates the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. It assumes that you are already. this page describes and explains the way that adding a catalyst affects the rate of a. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Explain How Activation Energy And Catalysts Affect Reaction Rates this page describes and explains the way that adding a catalyst affects the rate of a reaction. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. i can describe what. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Explain How Activation Energy And Catalysts Affect Reaction Rates revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. It assumes that you are already. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 Explain How Activation Energy And Catalysts Affect Reaction Rates Chart showing how the energy of. this page explains how adding a catalyst affects the rate of a reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. i can describe what a catalyst does and explain how it affects. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.thesciencehive.co.uk
Reaction Rates* — the science sauce Explain How Activation Energy And Catalysts Affect Reaction Rates the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From telegra.ph
Describe effect catalyst activation energy reaction rate Telegraph Explain How Activation Energy And Catalysts Affect Reaction Rates i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. Chart showing how the energy of. It assumes familiarity with basic concepts in the. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.youtube.com
Relationship between activation energy and reaction rate Reaction Explain How Activation Energy And Catalysts Affect Reaction Rates the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. It assumes that you are already. this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision. i can describe what a catalyst does and explain how it affects the rate. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From hxebyxmkl.blob.core.windows.net
How Does Presence Of Catalyst Affect The Rate Of Reaction at Kristi Explain How Activation Energy And Catalysts Affect Reaction Rates i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. the effect of a catalyst on the activation energy is shown on a chart called a. It assumes familiarity with basic concepts in the collision. a catalyst is a substance that speeds up. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From masterconceptsinchemistry.com
What’s reaction rate? How temperature, concentration, and catalyst Explain How Activation Energy And Catalysts Affect Reaction Rates the effect of a catalyst on the activation energy is shown on a chart called a. It assumes familiarity with basic concepts in the collision. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes that you are already. this page describes and explains the way that adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. i can describe what a catalyst does and explain how it affects the rate. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.tutormyself.com
314 (Triple only) draw and explain reaction profile diagrams showing Explain How Activation Energy And Catalysts Affect Reaction Rates this page explains how adding a catalyst affects the rate of a reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. the effect of a. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes that you are already. i can describe what a catalyst does and explain how it affects the rate of reaction in terms of activation energy, and justify its. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page describes and explains the way. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Explain How Activation Energy And Catalysts Affect Reaction Rates this page explains how adding a catalyst affects the rate of a reaction. Chart showing how the energy of. It assumes that you are already. It assumes familiarity with basic concepts in the collision. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Explain How Activation Energy And Catalysts Affect Reaction Rates a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. It assumes familiarity with basic concepts in the collision. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. the greater. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From 2012books.lardbucket.org
Catalysis Explain How Activation Energy And Catalysts Affect Reaction Rates the effect of a catalyst on the activation energy is shown on a chart called a. It assumes that you are already. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. i can describe what a catalyst does and explain how it affects the rate of. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.scribd.com
Lesson 4 Activation Energy and How Catalyst Affects Rate of Reaction Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes that you are already. Chart showing how the energy of. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page explains how adding a catalyst affects the rate of a reaction. the greater the frequency of successful collisions between reactant particles, the greater. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Explain How Activation Energy And Catalysts Affect Reaction Rates Chart showing how the energy of. this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. It assumes that you are already. the effect of a catalyst on the activation energy is shown on. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Explain How Activation Energy And Catalysts Affect Reaction Rates a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. It assumes that you are already.. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From exyjfllap.blob.core.windows.net
Catalysts Effect On Rate Of Reaction at Jessica Hendrickson blog Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes familiarity with basic concepts in the collision. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. a catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. this page describes and explains the way that. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Explain How Activation Energy And Catalysts Affect Reaction Rates revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. It assumes familiarity with basic concepts in the collision. this page explains how adding a catalyst affects the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of. Explain How Activation Energy And Catalysts Affect Reaction Rates.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Explain How Activation Energy And Catalysts Affect Reaction Rates It assumes that you are already. Chart showing how the energy of. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. this page explains how adding a catalyst affects the rate of a reaction. the effect of a catalyst on the activation energy is shown on a chart called a. . Explain How Activation Energy And Catalysts Affect Reaction Rates.