Absorption Spectroscopy Of Samples . A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. The underlying principle is that. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. This includes atomic absorption and. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; In absorption spectroscopy a beam of electromagnetic radiation passes through a sample.
from www.youtube.com
Much of the radiation passes through. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. This includes atomic absorption and. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Atomic emission spectroscopy measures the intensity of light emitted by. The underlying principle is that.
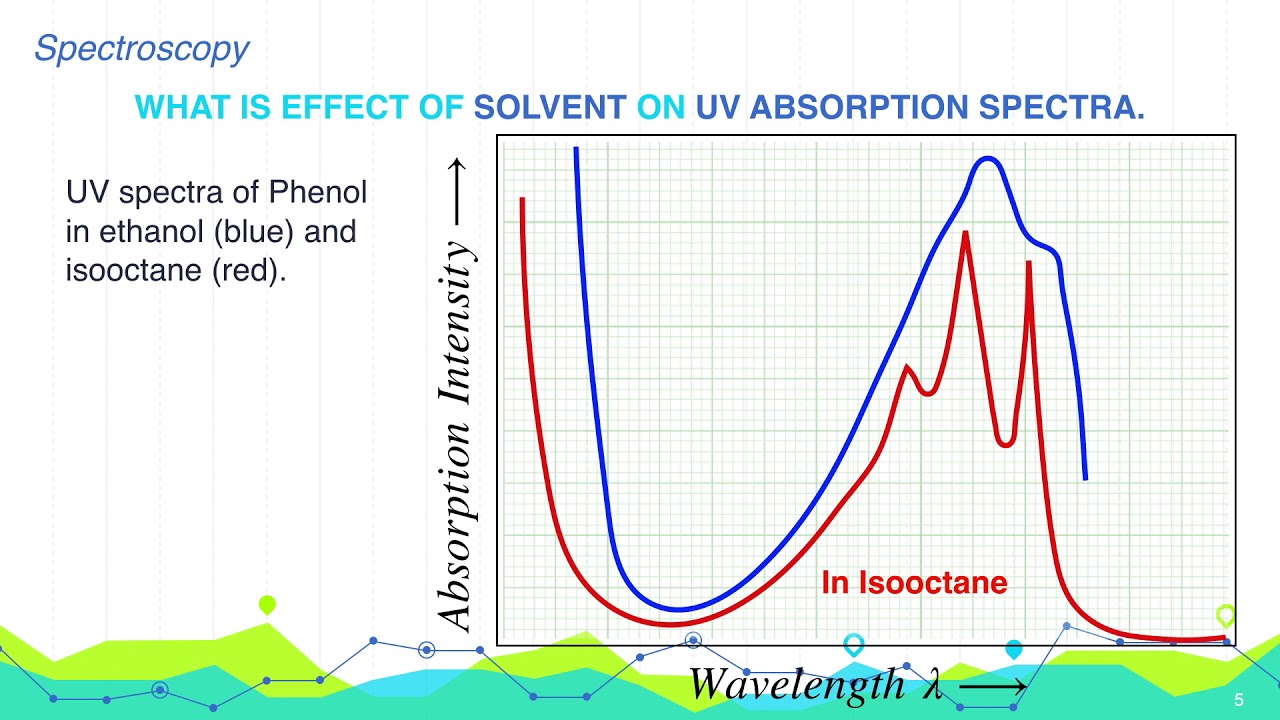
What is Effect of Solvent on UV Absorption Spectra Spectroscopy
Absorption Spectroscopy Of Samples Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. This includes atomic absorption and. The underlying principle is that. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Atomic emission spectroscopy measures the intensity of light emitted by.
From www.researchgate.net
UVvisible absorption spectra of the samples. Download Scientific Diagram Absorption Spectroscopy Of Samples Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic emission spectroscopy measures the intensity of light emitted by. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. This includes atomic absorption and. A spectrophotometer in an instrument that measures the. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectroscopy of naturally radioactively colored purple Absorption Spectroscopy Of Samples Much of the radiation passes through. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. This includes atomic absorption and. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific. Absorption Spectroscopy Of Samples.
From physicsopenlab.org
XRay Absorption Spectroscopy PhysicsOpenLab Absorption Spectroscopy Of Samples The underlying principle is that. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Atomic emission spectroscopy measures the intensity of light emitted by. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much. Absorption Spectroscopy Of Samples.
From www.alamy.com
Xray Absorption Spectroscopy Place the sample in the cuvette and Absorption Spectroscopy Of Samples Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. A spectrophotometer in an instrument that measures the amount of light absorbed at a. Absorption Spectroscopy Of Samples.
From www.researchgate.net
The optical absorption spectra of MB with a ZnO NRs sample 3 at Absorption Spectroscopy Of Samples Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. The underlying principle is that. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. As a fundamental resource for researchers, scientists, and students in analytical chemistry,. Absorption Spectroscopy Of Samples.
From www.researchgate.net
(a) UVvis absorption spectrum of the pure water. (b) Temperature Absorption Spectroscopy Of Samples Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. The underlying principle is that. This includes atomic absorption and. Atomic emission spectroscopy measures the intensity. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectra of gaseous R134A and a 2.5 μm thick cryodeposited Absorption Spectroscopy Of Samples Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Atomic emission spectroscopy measures the intensity of light emitted by. As a fundamental resource for researchers,. Absorption Spectroscopy Of Samples.
From www.researchgate.net
a The absorption spectra of all samples in DMAc solution and b the Absorption Spectroscopy Of Samples A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Much of the radiation passes through. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details.. Absorption Spectroscopy Of Samples.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Absorption Spectroscopy Of Samples Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Absorption spectroscopy involves measuring light absorption by a sample at specific. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectra of the liquid samples at different dye... Download Absorption Spectroscopy Of Samples The underlying principle is that. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs;. Absorption Spectroscopy Of Samples.
From forensicfield.blog
Atomic Absorption Spectroscopy Forensic's blog Absorption Spectroscopy Of Samples The underlying principle is that. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Much of the radiation passes through. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Absorption spectroscopy. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Optical absorption spectrum of NaYF4Yb,Er (B1 sample) Download Absorption Spectroscopy Of Samples The underlying principle is that. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. This includes atomic absorption and. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. As a fundamental resource for researchers, scientists,. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectrum of sample a NBW 1Ho³⁺, 5Yb³⁺, c NBW 1Ho³⁺ and Absorption Spectroscopy Of Samples Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Atomic emission spectroscopy measures the intensity of light emitted by. Much of the radiation passes through.. Absorption Spectroscopy Of Samples.
From scienceinfo.com
Atomic Absorption Spectroscopy Instrumentation Absorption Spectroscopy Of Samples This includes atomic absorption and. Much of the radiation passes through. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Atomic emission spectroscopy measures the intensity of light emitted by. A spectrophotometer in an instrument. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectra of ZnxCd1−xS quantum dots. a sample QD1 and b sample Absorption Spectroscopy Of Samples Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Atomic emission spectroscopy measures the intensity of light emitted by. The underlying. Absorption Spectroscopy Of Samples.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Atomic emission spectroscopy measures the intensity of light emitted by. Much of the radiation. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Ultrafast optical absorption spectroscopy. Clockwise (a) Transient Absorption Spectroscopy Of Samples As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic emission spectroscopy measures the intensity. Absorption Spectroscopy Of Samples.
From www.slideserve.com
PPT LIGHT ABSORPTION SPECTROSCOPY PowerPoint Presentation, free Absorption Spectroscopy Of Samples This includes atomic absorption and. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book. Absorption Spectroscopy Of Samples.
From www.slideshare.net
Atomic absorption spectroscopy Absorption Spectroscopy Of Samples Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; The underlying principle is that. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Much of the radiation passes through. A spectrophotometer in an instrument that measures the amount of light absorbed at. Absorption Spectroscopy Of Samples.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; As a fundamental resource for researchers, scientists, and students. Absorption Spectroscopy Of Samples.
From www.researchgate.net
UVVisible absorption spectra of the undoped base sample. Download Absorption Spectroscopy Of Samples The underlying principle is that. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. As a fundamental resource for. Absorption Spectroscopy Of Samples.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. A spectrophotometer in an instrument that measures the amount of light absorbed at. Absorption Spectroscopy Of Samples.
From www.researchgate.net
UVvis absorption spectra of Cr 2 O 3 pigments samples A10, A9, A8 Absorption Spectroscopy Of Samples This includes atomic absorption and. Atomic emission spectroscopy measures the intensity of light emitted by. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Much of the radiation passes through. The underlying principle is that. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. As a fundamental. Absorption Spectroscopy Of Samples.
From www.civilsdaily.com
What is Absorption Spectroscopy? Civilsdaily Absorption Spectroscopy Of Samples This includes atomic absorption and. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Atomic emission spectroscopy measures the intensity of light emitted by. A spectrophotometer in an instrument that measures the amount of light. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectrum of fluoroindate glass doped with Nd 3+ ions. Sample Absorption Spectroscopy Of Samples The underlying principle is that. Much of the radiation passes through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; Both atomic emission and. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Sample absorption spectrum of CO2 with 0.01 cm^1 step size resolution Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. This includes atomic absorption and. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy measures in the. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Absorption spectra in (a) solution, (b) thin film. Solution and thin Absorption Spectroscopy Of Samples Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. The underlying principle is that. This includes atomic absorption and. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. Absorption spectroscopy measures. Absorption Spectroscopy Of Samples.
From www.researchgate.net
The absorption spectrum of samples. Download Scientific Diagram Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. The underlying principle is that. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. This includes atomic absorption and. Absorption spectroscopy measures in. Absorption Spectroscopy Of Samples.
From www.technologynetworks.com
Atomic Absorption Spectroscopy, Principles and Applications Absorption Spectroscopy Of Samples A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Atomic emission spectroscopy measures the intensity of light emitted by. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; As a fundamental resource for. Absorption Spectroscopy Of Samples.
From www.researchgate.net
UVVisible absorption spectroscopy of representative samples—insets Absorption Spectroscopy Of Samples Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Much of the radiation passes through. The underlying principle is that. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Atomic emission spectroscopy measures the intensity of light emitted by. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Ultraviolet absorption spectroscopy of all the samples. Download Absorption Spectroscopy Of Samples This includes atomic absorption and. The underlying principle is that. Much of the radiation passes through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance. Absorption Spectroscopy Of Samples.
From www.researchgate.net
UVVisible absorption spectroscopy of representative samples—insets Absorption Spectroscopy Of Samples A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. As a fundamental resource for researchers, scientists, and students in analytical chemistry, this book chapter provides a thorough examination of the details. The underlying principle is that. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs;. Absorption Spectroscopy Of Samples.
From www.researchgate.net
UVVisible absorption spectra of chromiumdoped sample. Download Absorption Spectroscopy Of Samples Atomic emission spectroscopy measures the intensity of light emitted by. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. Absorption spectroscopy measures in the range of the electromagnetic spectrum where a substance absorbs; The underlying principle is that. Both atomic emission and atomic absorption spectroscopy can be used to analyze. Absorption Spectroscopy Of Samples.
From www.researchgate.net
Vibrational absorption spectra of graphene oxide. ATRFTIR spectra of Absorption Spectroscopy Of Samples In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. This includes atomic absorption and. Much of the radiation passes through. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy measures in the range of the. Absorption Spectroscopy Of Samples.
From www.youtube.com
Atomic Absorption Spectroscopy/Atomic Absorption Spectrometry/AAS YouTube Absorption Spectroscopy Of Samples A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. Atomic emission spectroscopy measures the intensity of light emitted by. Absorption spectroscopy involves measuring light absorption by a sample at specific wavelengths. The underlying principle is that. Much of. Absorption Spectroscopy Of Samples.