Does Gases Move From High Pressure To Low Pressure . And the bigger the difference between the pressures, the faster the air will move from the high to the. Think of a blunt body immersed in a flow. This demo of bernoulli's principle appears to show gas. Particles of gas accelerate towards lower pressure. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. They can move from a low pressure region to high pressure. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. My understanding is that gases move from high to low partial pressure independent of other gases. Consider the streamline that starts a long. Particle do not always move from high pressure to low pressure.
from www.slideserve.com
When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. And the bigger the difference between the pressures, the faster the air will move from the high to the. They can move from a low pressure region to high pressure. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Think of a blunt body immersed in a flow. Consider the streamline that starts a long. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. Particle do not always move from high pressure to low pressure. This demo of bernoulli's principle appears to show gas. My understanding is that gases move from high to low partial pressure independent of other gases.
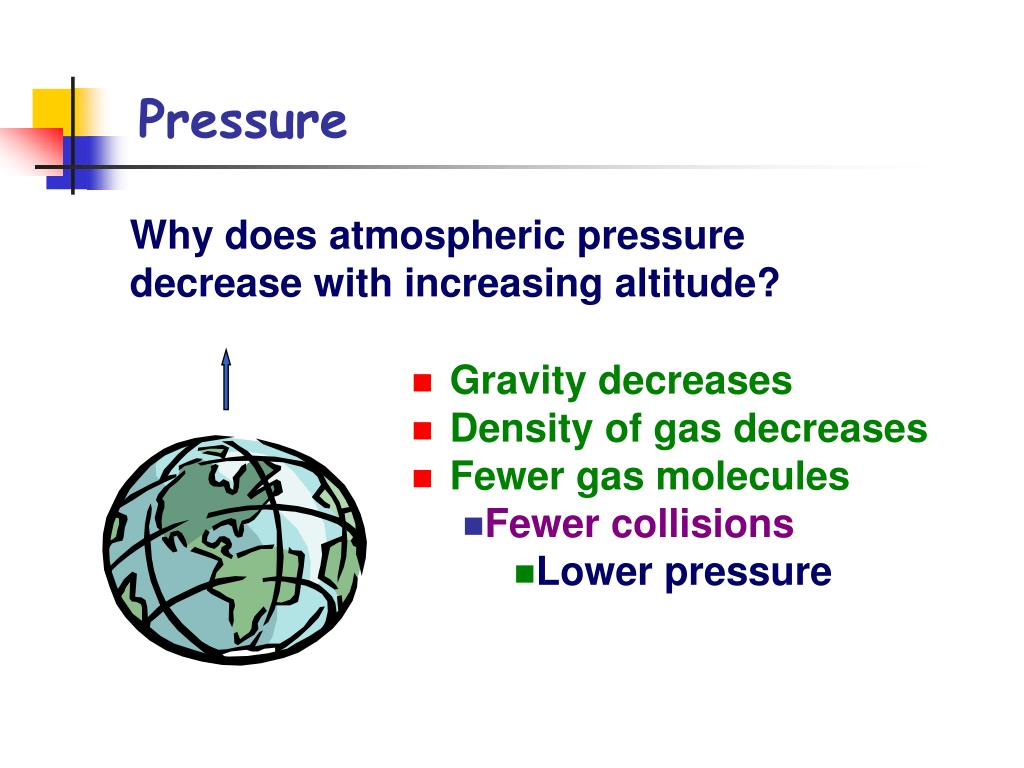
PPT Unit 6 Gases, Phase Changes and Introduction to Thermochemistry
Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. And the bigger the difference between the pressures, the faster the air will move from the high to the. Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. My understanding is that gases move from high to low partial pressure independent of other gases. Particle do not always move from high pressure to low pressure. Think of a blunt body immersed in a flow. Consider the streamline that starts a long. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). This demo of bernoulli's principle appears to show gas. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. Particles of gas accelerate towards lower pressure. They can move from a low pressure region to high pressure.
From sciencenotes.org
Real Gas vs Ideal Gas Does Gases Move From High Pressure To Low Pressure This demo of bernoulli's principle appears to show gas. They can move from a low pressure region to high pressure. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. And the bigger the difference between the pressures, the faster the air will move from the high to the. Yes, gas flow. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Ocean Circulation PowerPoint Presentation, free download ID5948883 Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. Think of a blunt body immersed in a flow. They can move from a low pressure region to high pressure. My understanding is that gases move from high to low partial pressure independent of other gases. Particles of gas accelerate towards lower pressure. Consider the streamline that starts a. Does Gases Move From High Pressure To Low Pressure.
From www.thegeographeronline.net
Weather and Climate THE GEOGRAPHER ONLINE Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Think of a blunt body immersed in a flow. Consider the streamline that starts a long. Gas pressure is caused by molecules moving at speed and hitting the walls of the. Does Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Does Gases Move From High Pressure To Low Pressure Particles of gas accelerate towards lower pressure. This demo of bernoulli's principle appears to show gas. They can move from a low pressure region to high pressure. Consider the streamline that starts a long. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Yes, gas flow from high to. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Unit 6 Gases, Phase Changes and Introduction to Thermochemistry Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). They can move from a low pressure region to high pressure. Particles of gas accelerate towards lower pressure. And the bigger the difference between the pressures, the faster the air will move from the high to the. Consider the streamline. Does Gases Move From High Pressure To Low Pressure.
From www.fox6now.com
The physics behind high and low pressure FOX6 Milwaukee Does Gases Move From High Pressure To Low Pressure Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. And the bigger the difference between the pressures, the faster the air will move from the high to the. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. They can move. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT High and Low Pressure Systems Weather Systems Unit PowerPoint Does Gases Move From High Pressure To Low Pressure My understanding is that gases move from high to low partial pressure independent of other gases. Particles of gas accelerate towards lower pressure. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. They can move from a low pressure region to high pressure. And the bigger the difference between the pressures,. Does Gases Move From High Pressure To Low Pressure.
From uk.ixl.com
IXL How does particle motion affect gas pressure? 7th grade science Does Gases Move From High Pressure To Low Pressure This demo of bernoulli's principle appears to show gas. And the bigger the difference between the pressures, the faster the air will move from the high to the. My understanding is that gases move from high to low partial pressure independent of other gases. Gas pressure is caused by molecules moving at speed and hitting the walls of the container. Does Gases Move From High Pressure To Low Pressure.
From learnbps.bismarckschools.org
States of Matter (Book) Behavior of Gases Learnbps Does Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high pressure. Particles of gas accelerate towards lower pressure. Consider the streamline that starts a long. Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. And the bigger the difference between the pressures, the faster the air will move from. Does Gases Move From High Pressure To Low Pressure.
From www.youtube.com
Gases Pressure. YouTube Does Gases Move From High Pressure To Low Pressure Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. And the bigger the difference between the pressures, the faster the air will move from the high to the. Consider the streamline that starts a long. My understanding is that gases move from high to. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT The Restless Atmosphere PowerPoint Presentation, free download Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. Particles of gas accelerate towards lower pressure. This demo of bernoulli's principle appears to show gas. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Consider the streamline that starts a long. Think of a blunt body immersed in a. Does Gases Move From High Pressure To Low Pressure.
From pilotinstitute.com
High vs. LowPressure Systems Explained Pilot Institute Does Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high pressure. Think of a blunt body immersed in a flow. This demo of bernoulli's principle appears to show gas. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Classical physics states that a gas will only flow from a. Does Gases Move From High Pressure To Low Pressure.
From pilotinstitute.com
High vs. LowPressure Systems Explained Pilot Institute Does Gases Move From High Pressure To Low Pressure My understanding is that gases move from high to low partial pressure independent of other gases. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Yes, gas flow from high to. Does Gases Move From High Pressure To Low Pressure.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. They can move from a low pressure region to high pressure. My understanding is that gases move from high to low partial pressure independent of other gases. And the. Does Gases Move From High Pressure To Low Pressure.
From www.istockphoto.com
High Pressure And Low Pressure Illustration Stock Illustration Does Gases Move From High Pressure To Low Pressure And the bigger the difference between the pressures, the faster the air will move from the high to the. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. Particle do not. Does Gases Move From High Pressure To Low Pressure.
From studylib.net
High Pressure, Low Pressure and Fronts Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). My understanding is that gases move from high to low partial pressure independent of other gases. Particles of gas accelerate towards lower pressure. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow. Does Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Does Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high pressure. Particle do not always move from high pressure to low pressure. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. Consider the streamline that starts a long. And the bigger the difference between. Does Gases Move From High Pressure To Low Pressure.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. And the bigger the difference between the pressures, the faster the air will move from. Does Gases Move From High Pressure To Low Pressure.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Gases Move From High Pressure To Low Pressure Consider the streamline that starts a long. Particle do not always move from high pressure to low pressure. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. When a. Does Gases Move From High Pressure To Low Pressure.
From www.expii.com
Real vs. Ideal Gases — Comparison & Importance Expii Does Gases Move From High Pressure To Low Pressure Think of a blunt body immersed in a flow. They can move from a low pressure region to high pressure. My understanding is that gases move from high to low partial pressure independent of other gases. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Yes, gas flow from. Does Gases Move From High Pressure To Low Pressure.
From www.youtube.com
Calculating Gas Pressure Pressure in the Gas Laws CLEAR & SIMPLE Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). They can move from a low pressure region to high pressure. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. And the bigger the difference between the pressures, the faster the. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Weather Vocabulary Terms PowerPoint Presentation, free download Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Particles of gas accelerate towards lower pressure. Think of a blunt body immersed in a flow. Classical physics states that a gas. Does Gases Move From High Pressure To Low Pressure.
From ar.inspiredpencil.com
High And Low Pressure Systems Does Gases Move From High Pressure To Low Pressure Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). And the bigger the difference between the pressures, the faster the air will move from the high to the. Think of a blunt body immersed in a flow. Classical physics states that a gas will only flow from a region. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Atmospheric Motion PowerPoint Presentation, free download ID Does Gases Move From High Pressure To Low Pressure Consider the streamline that starts a long. And the bigger the difference between the pressures, the faster the air will move from the high to the. Think of a blunt body immersed in a flow. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. This demo of bernoulli's principle appears to. Does Gases Move From High Pressure To Low Pressure.
From saylordotorg.github.io
Gases and Pressure Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. Particles of gas accelerate towards lower pressure. They can move from a low pressure region to high pressure. Consider the streamline that starts a long. This demo of bernoulli's principle appears to show gas. Think of a blunt body immersed in a flow. And the bigger the difference between. Does Gases Move From High Pressure To Low Pressure.
From www.myarklamiss.com
HIGH vs. LOW What are the impacts of weather pressures? KTVE Does Gases Move From High Pressure To Low Pressure Particles of gas accelerate towards lower pressure. Particle do not always move from high pressure to low pressure. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or. Does Gases Move From High Pressure To Low Pressure.
From saylordotorg.github.io
Gases Does Gases Move From High Pressure To Low Pressure When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Particles of gas accelerate towards lower pressure. They can move from a low pressure region to high pressure. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). And the bigger the. Does Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Does Gases Move From High Pressure To Low Pressure Particle do not always move from high pressure to low pressure. My understanding is that gases move from high to low partial pressure independent of other gases. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Think of a blunt body immersed in a flow. Consider the streamline that starts a. Does Gases Move From High Pressure To Low Pressure.
From edurev.in
Gaseous Introduction (Part 1) Chemistry Notes EduRev Does Gases Move From High Pressure To Low Pressure And the bigger the difference between the pressures, the faster the air will move from the high to the. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6918783 Does Gases Move From High Pressure To Low Pressure Particles of gas accelerate towards lower pressure. They can move from a low pressure region to high pressure. Consider the streamline that starts a long. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. This demo of bernoulli's principle appears to show gas. Yes,. Does Gases Move From High Pressure To Low Pressure.
From www.youtube.com
Does fluid always flow from high pressure to low pressure? Quick Does Gases Move From High Pressure To Low Pressure Yes, gas flow from high to low pressure can be reversed by creating a pressure gradient in the opposite direction. Think of a blunt body immersed in a flow. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Particle do not always move from high pressure to low pressure. This demo. Does Gases Move From High Pressure To Low Pressure.
From www.logiota.com
Deviation from Ideal Gas Behavior States of Matter Physical Does Gases Move From High Pressure To Low Pressure My understanding is that gases move from high to low partial pressure independent of other gases. This demo of bernoulli's principle appears to show gas. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure sensor). Particle do not always move from high pressure to low pressure. Consider the streamline that. Does Gases Move From High Pressure To Low Pressure.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning Does Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high pressure. And the bigger the difference between the pressures, the faster the air will move from the high to the. This demo of bernoulli's principle appears to show gas. Consider the streamline that starts a long. My understanding is that gases move from high to low partial pressure independent of. Does Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free Does Gases Move From High Pressure To Low Pressure This demo of bernoulli's principle appears to show gas. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. Particles of gas accelerate towards lower pressure. Gas pressure is caused by molecules moving at speed and hitting the walls of the container (or your pressure. Does Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Does Gases Move From High Pressure To Low Pressure My understanding is that gases move from high to low partial pressure independent of other gases. When a fluid encounters high pressure, it experiences greater forces opposing its movement, leading to reduced flow rates. Classical physics states that a gas will only flow from a region of high to one of low pressure and, like water, won't run uphill. And. Does Gases Move From High Pressure To Low Pressure.