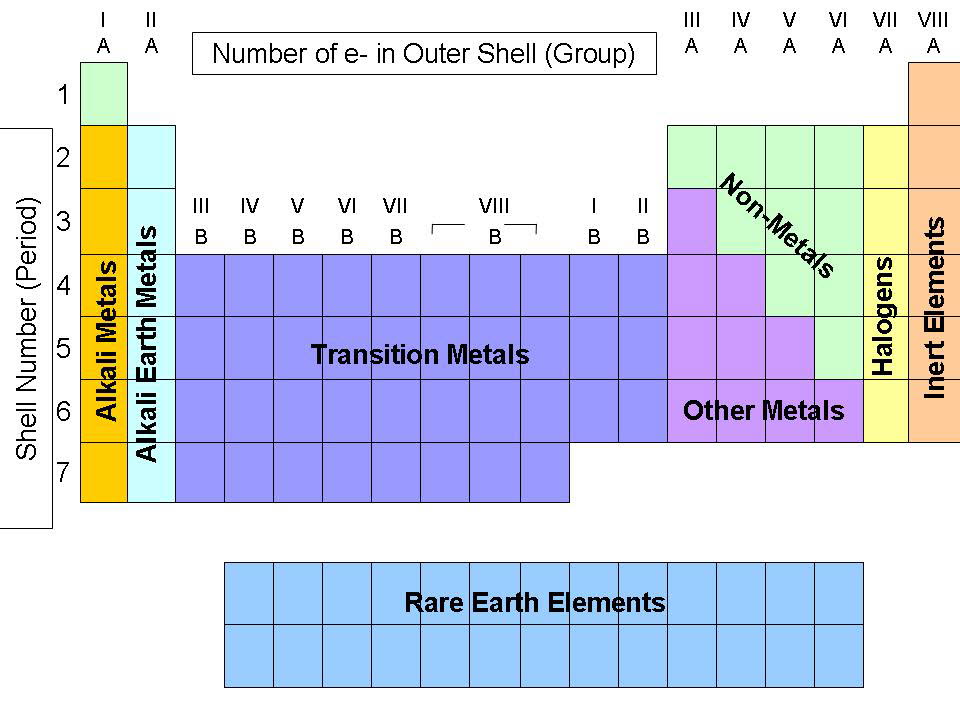
What Metals Are In Group 1 . The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each have. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The alkali metals make up group 1 of the periodic table. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 elements are all soft, reactive metals with low melting points. They form alkaline solutions when they react with water. The group 1 elements are known as the alkali metals. They react with water to produce an alkaline metal hydroxide solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,.
from new-periodic11.blogspot.com
The alkali metals share similar characteristic chemical properties because they each have. The alkali metals make up group 1 of the periodic table. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. They react with water to produce an alkaline metal hydroxide solution. The alkali metals share similar. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1 elements are known as the alkali metals. The group 1 elements are all soft, reactive metals with low melting points. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. They form alkaline solutions when they react with water.
NEW ALKALI METALS IN THE PERIODIC TABLE OF ELEMENTS Periodic
What Metals Are In Group 1 The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. They react with water to produce an alkaline metal hydroxide solution. The alkali metals share similar. They form alkaline solutions when they react with water. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 elements are known as the alkali metals. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals make up group 1 of the periodic table. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali metals share similar characteristic chemical properties because they each have.
From www.sliderbase.com
Group 1 Presentation Chemistry What Metals Are In Group 1 The alkali metals share similar. The group 1 elements are known as the alkali metals. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They form alkaline solutions when they react with water. The group 1 elements are. What Metals Are In Group 1.
From utedzz.blogspot.com
Periodic Table Group 1 Alkali Metals Periodic Table Timeline What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The alkali metals share similar. They form alkaline solutions when they react with water. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and. What Metals Are In Group 1.
From theconversation.com
Understanding the periodic table through the lens of the volatile Group What Metals Are In Group 1 They react with water to produce an alkaline metal hydroxide solution. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 elements are known as the alkali metals. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first. What Metals Are In Group 1.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) What Metals Are In Group 1 They react with water to produce an alkaline metal hydroxide solution. The group 1 elements are all soft, reactive metals with low melting points. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali metals make up group 1 of the periodic table. The alkali metals share similar. The group 1 elements are known as the alkali. What Metals Are In Group 1.
From sciencenotes.org
Main Group Elements Definition and Importance What Metals Are In Group 1 The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals share similar. The group 1 metals are lithium, sodium, potassium, rubidium, caesium. What Metals Are In Group 1.
From www.youtube.com
Let's learn Group 1 elements alkali metals in periodic table using What Metals Are In Group 1 This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The alkali metals make up group 1 of the periodic table. They react with water to produce an alkaline metal hydroxide solution. The group 1 metals are lithium, sodium,. What Metals Are In Group 1.
From periodictableguide.com
Periodic table Groups Explained !! (With 118 Group Names) What Metals Are In Group 1 They react with water to produce an alkaline metal hydroxide solution. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are known as the alkali metals. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 metals are lithium, sodium,. What Metals Are In Group 1.
From www.pathwaystochemistry.com
Common Groups of Elements Pathways to Chemistry What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each have. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. This family consists of the elements. What Metals Are In Group 1.
From chemassist.blogspot.com
ChemAssist Groups of the Periodic Table What Metals Are In Group 1 The alkali metals share similar characteristic chemical properties because they each have. They form alkaline solutions when they react with water. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. They react with water to produce an alkaline metal hydroxide solution. The group 1 elements are all soft, reactive. What Metals Are In Group 1.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The alkali metals make up group 1 of the periodic table. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. They form alkaline solutions when they react with water. The group 1 metals are lithium, sodium,. What Metals Are In Group 1.
From www.slideshare.net
The periodic table What Metals Are In Group 1 The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. They form alkaline solutions when they react with water. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are all soft, reactive. What Metals Are In Group 1.
From www.sciencelearn.org.nz
Periodic table of elements — Science Learning Hub What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The alkali metals share similar. The group 1 elements are known as the alkali metals. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of. What Metals Are In Group 1.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals What Metals Are In Group 1 The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal hydroxide solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali metals share similar. What Metals Are In Group 1.
From www.livescience.com
How the Periodic Table groups the elements Live Science What Metals Are In Group 1 The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each have. They form alkaline solutions when they react with water. The group 1 elements are known as the alkali metals. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1 elements, also known as the alkali metals, all react vigorously. What Metals Are In Group 1.
From www.youtube.com
The Alkali Metals Group 1 YouTube What Metals Are In Group 1 They form alkaline solutions when they react with water. The alkali metals share similar characteristic chemical properties because they each have. The alkali metals share similar. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The alkali metals make up group 1 of the periodic. What Metals Are In Group 1.
From www.breakingatom.com
Group 1 The Alkali Metals What Metals Are In Group 1 The group 1 elements are known as the alkali metals. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They react with water to produce an alkaline metal hydroxide solution. The group 1 elements. What Metals Are In Group 1.
From www.ck12.org
Families and Periods of the Periodic Table CK12 Foundation What Metals Are In Group 1 The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each have. They react with water to produce an alkaline metal hydroxide solution. The group 1 elements are known as the alkali metals. The group 1 elements are all soft, reactive metals with low melting points. They form alkaline solutions when they react with water.. What Metals Are In Group 1.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic What Metals Are In Group 1 This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They form alkaline solutions when they react with water. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are all soft, reactive metals with low melting points. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are. What Metals Are In Group 1.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals What Metals Are In Group 1 They form alkaline solutions when they react with water. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals make up group 1 of the periodic table. The group 1 elements are known as the alkali metals. They react with water to produce an alkaline metal hydroxide solution. The group 1 metals are lithium,. What Metals Are In Group 1.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions What Metals Are In Group 1 The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The alkali metals make up group 1 of the periodic table. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali metals share similar. They react with water to produce an alkaline metal. What Metals Are In Group 1.
From www.periodictableprintable.com
Description Of Metals On The Periodic Table Periodic Table Printable What Metals Are In Group 1 The alkali metals share similar. The alkali metals make up group 1 of the periodic table. They react with water to produce an alkaline metal hydroxide solution. They form alkaline solutions when they react with water. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are all soft, reactive metals with low melting. What Metals Are In Group 1.
From www.thoughtco.com
Properties of Periodic Table of Element Groups What Metals Are In Group 1 This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They react with water to produce an alkaline metal hydroxide solution. The alkali metals make up group 1 of the periodic table. The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each have. They form alkaline solutions when they react with water. The. What Metals Are In Group 1.
From quizlet.com
Periodic Table of Elements with Group Names Diagram Quizlet What Metals Are In Group 1 The alkali metals share similar. The group 1 elements are known as the alkali metals. They form alkaline solutions when they react with water. They react with water to produce an alkaline metal hydroxide solution. The alkali metals make up group 1 of the periodic table. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they. What Metals Are In Group 1.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation, free What Metals Are In Group 1 They react with water to produce an alkaline metal hydroxide solution. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. They form alkaline solutions when they react with water. The alkali metals share similar. The alkali metals share similar characteristic chemical properties because they each. What Metals Are In Group 1.
From new-periodic11.blogspot.com
NEW ALKALI METALS IN THE PERIODIC TABLE OF ELEMENTS Periodic What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The alkali metals make up group 1 of the periodic table. They form alkaline solutions when they react with water. The alkali metals share similar. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the. What Metals Are In Group 1.
From elchoroukhost.net
Alkali Metals Periodic Table Properties Elcho Table What Metals Are In Group 1 They form alkaline solutions when they react with water. The alkali metals share similar characteristic chemical properties because they each have. The alkali metals share similar. The group 1 elements are all soft, reactive metals with low melting points. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. This. What Metals Are In Group 1.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica What Metals Are In Group 1 The group 1 elements are all soft, reactive metals with low melting points. The group 1 elements are known as the alkali metals. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They react with water to produce an alkaline metal hydroxide solution. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are. What Metals Are In Group 1.
From www.youtube.com
Group 1 Metal Periodic table YouTube What Metals Are In Group 1 This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They react with water to produce an alkaline metal hydroxide solution. The alkali metals make up group 1 of the periodic table. They form alkaline solutions when they react with water. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements are known. What Metals Are In Group 1.
From www.priyamstudycentre.com
Alkali Metals Periodic Table Elements What Metals Are In Group 1 The alkali metals share similar. The alkali metals make up group 1 of the periodic table. The alkali metals share similar characteristic chemical properties because they each have. The group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The. What Metals Are In Group 1.
From www.sliderbase.com
Group 1&2 Presentation Chemistry What Metals Are In Group 1 The alkali metals share similar characteristic chemical properties because they each have. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The alkali metals share similar. The group 1 elements are known as the alkali metals. They form alkaline solutions when they react with water.. What Metals Are In Group 1.
From dashamlav.com
Periodic Table Groups Names and Properties What Metals Are In Group 1 The alkali metals share similar characteristic chemical properties because they each have. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. They react with water to produce an alkaline metal hydroxide solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The alkali. What Metals Are In Group 1.
From www.goodscience.com.au
The Periodic Table Good Science What Metals Are In Group 1 This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. They react with water to produce an alkaline metal hydroxide solution. They form alkaline solutions when they react with water. The group 1 elements are known as the alkali metals. The alkali metals share similar. The alkali metals make up group 1 of the periodic table. The group 1. What Metals Are In Group 1.
From period-faqs.com
Where Are The Alkali Metals On The Periodic Table What Metals Are In Group 1 The alkali metals share similar. The group 1 elements are all soft, reactive metals with low melting points. The group 1 elements are known as the alkali metals. They react with water to produce an alkaline metal hydroxide solution. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of. What Metals Are In Group 1.
From www.thesciencehive.co.uk
The Periodic Table (AQA) — the science hive What Metals Are In Group 1 They form alkaline solutions when they react with water. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The group 1 elements are all soft, reactive metals with low melting points. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1. What Metals Are In Group 1.
From www.thoughtco.com
Main Group Elements Definition What Metals Are In Group 1 They react with water to produce an alkaline metal hydroxide solution. This family consists of the elements lithium, sodium, potassium, rubidium, cesium,. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. The group 1 elements, also known as the alkali metals, all react vigorously with. What Metals Are In Group 1.