Chemistry Buffer Lab Report . buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. Designing and preparing a buffer. Explain why the buffer capacity depends on the concentration of. compare the results of the ph sensitivity data in parts a and c. Calculate the individual concentrations and. [acid] + [base] = 0 m. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Solutions by utilizing a ph electrode. show calculations for the assigned buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small.
from www.studocu.com
Solutions by utilizing a ph electrode. Calculate the individual concentrations and. show calculations for the assigned buffer. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. compare the results of the ph sensitivity data in parts a and c. The purpose of this experiment is to test a buffer’s behavior and capacity with different. [acid] + [base] = 0 m. Explain why the buffer capacity depends on the concentration of. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Designing and preparing a buffer.
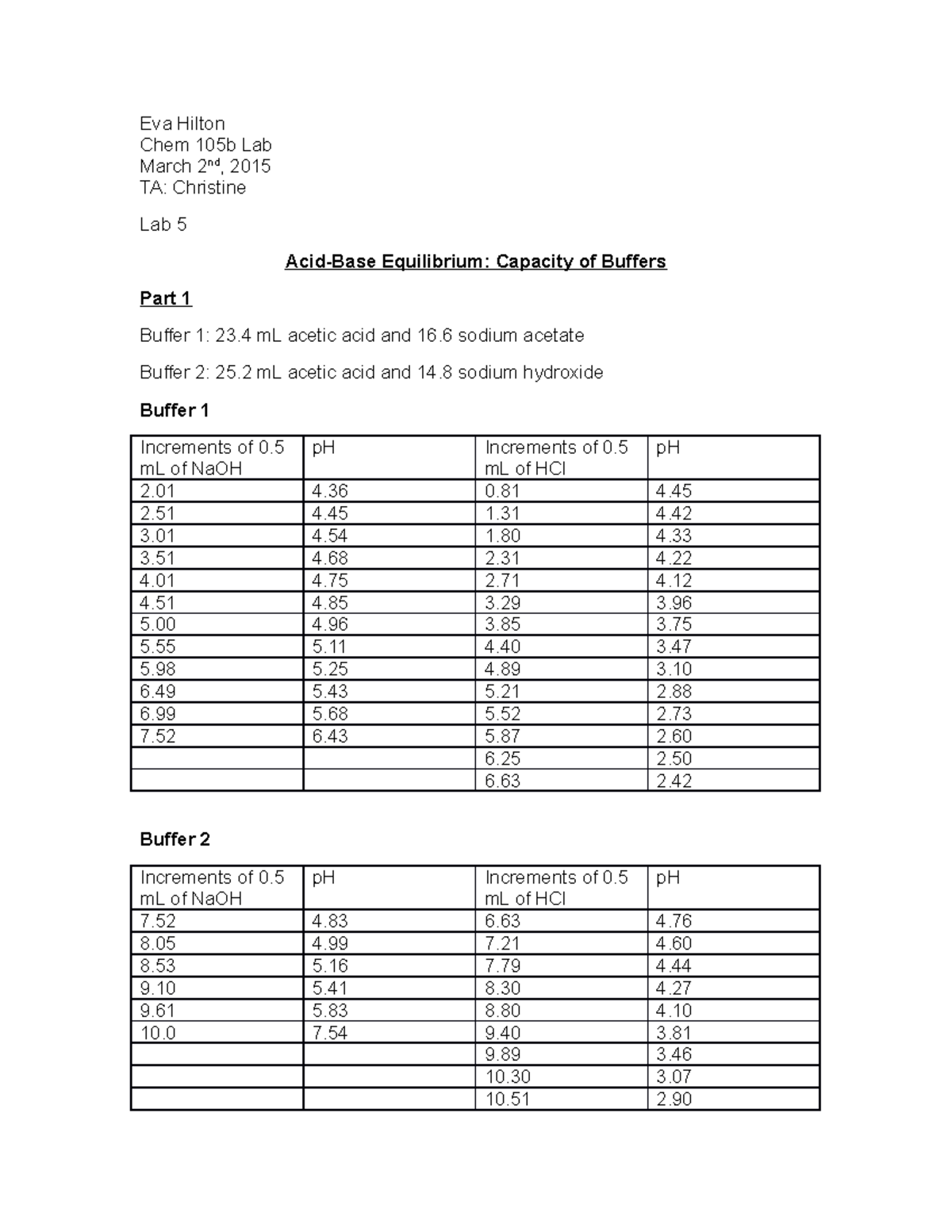
Lab 5 chem 105b buffers lab solutions Eva Hilton Chem 105b Lab
Chemistry Buffer Lab Report Solutions by utilizing a ph electrode. Designing and preparing a buffer. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. Explain why the buffer capacity depends on the concentration of. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Calculate the individual concentrations and. Solutions by utilizing a ph electrode. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. show calculations for the assigned buffer. compare the results of the ph sensitivity data in parts a and c. [acid] + [base] = 0 m.
From www.studocu.com
Buffers Lab Report Complete the below pages and submit them to your Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. Solutions by utilizing a ph electrode. [acid] + [base] = 0 m. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Designing and preparing a buffer. . Chemistry Buffer Lab Report.
From www.studocu.com
Report 2 Group 3 CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND Chemistry Buffer Lab Report The purpose of this experiment is to test a buffer’s behavior and capacity with different. Solutions by utilizing a ph electrode. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. buffers are most effective at protecting against ph change when they have similar concentrations of. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Chemistry Buffer Lab Report Explain why the buffer capacity depends on the concentration of. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Calculate the individual concentrations and. show calculations for the assigned buffer. The purpose of this experiment is to test a buffer’s behavior and capacity with different. . Chemistry Buffer Lab Report.
From www.studocu.com
Chem Lab report 3 3 3 3 Experiment 10. Hydrolysis and Buffers Chemistry Buffer Lab Report show calculations for the assigned buffer. Calculate the individual concentrations and. Explain why the buffer capacity depends on the concentration of. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. [acid] + [base] = 0 m. The purpose of this experiment is to test a buffer’s behavior and. Chemistry Buffer Lab Report.
From www.studocu.com
Chem 2360 lab report 2 Lab Report II Experiment 2 Buffers, Buffer Chemistry Buffer Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Designing and preparing a buffer. show calculations for the assigned buffer. compare the results of the ph sensitivity data in parts a and c. Calculate the individual concentrations and. Solutions by utilizing a ph electrode. The. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 2 Chem lab Lab 2 Chem lab CHEMISTRY LABORATORY REPORT Chemistry Buffer Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Solutions by utilizing a ph electrode. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. The purpose of this experiment is to test a buffer’s behavior and. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 11 Buffers LAB Lab 11 Examination of Buffer Solutions Chemistry Buffer Lab Report Explain why the buffer capacity depends on the concentration of. Calculate the individual concentrations and. Designing and preparing a buffer. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. show calculations for the assigned buffer. Solutions by utilizing a ph electrode. The dissociation of a weak. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 7 Buffers lab report Gianna Puopolo Lab Partner Nila Murugan Chemistry Buffer Lab Report [acid] + [base] = 0 m. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Calculate the individual concentrations and. show calculations for the assigned buffer. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Solutions by utilizing a ph electrode. compare the. Chemistry Buffer Lab Report.
From www.studocu.com
Experiment 12 LAB Report Abstract H 2 PO 4 /HPO 4 2 buffer system Chemistry Buffer Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. The purpose of this experiment is to test a buffer’s behavior and capacity with different. a buffer solution consists. Chemistry Buffer Lab Report.
From www.studocu.com
Lab report 2 CHEM271 EXPERIMENT 2 BUFFERS AND BIOCHEMICAL ANALYSIS Chemistry Buffer Lab Report The purpose of this experiment is to test a buffer’s behavior and capacity with different. Calculate the individual concentrations and. [acid] + [base] = 0 m. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. • investigate the nature of buffer solutions with respect to ph, buffer region,. Chemistry Buffer Lab Report.
From www.studocu.com
Buffer LAB Report Purpose The purpose of this experiment is to test Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. Designing and preparing a buffer. show calculations for the assigned buffer. compare the results of the ph sensitivity data in parts a and c. Explain why the buffer capacity depends on the concentration of. a buffer solution. Chemistry Buffer Lab Report.
From www.studocu.com
Chemistry Laboratory Report 2 CHEMISTRY LABORATORY REPORT EXPERIMENT Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. Calculate the individual concentrations and. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. [acid] + [base] = 0 m. The purpose of this experiment is to test a buffer’s behavior. Chemistry Buffer Lab Report.
From www.studocu.com
Chem 106 lab report 14 Experiment 14 Acids, Bases, Salts, and Chemistry Buffer Lab Report Explain why the buffer capacity depends on the concentration of. The purpose of this experiment is to test a buffer’s behavior and capacity with different. compare the results of the ph sensitivity data in parts a and c. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and. Chemistry Buffer Lab Report.
From www.studocu.com
Buffer Lab report summary Section______________ CHM115L Buffers Lab Chemistry Buffer Lab Report a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. compare the results of the ph sensitivity data in parts a and c. • investigate the nature. Chemistry Buffer Lab Report.
From www.studocu.com
Working with Buffers chemistry lab Working with Buffers Purpose The Chemistry Buffer Lab Report Calculate the individual concentrations and. The purpose of this experiment is to test a buffer’s behavior and capacity with different. compare the results of the ph sensitivity data in parts a and c. [acid] + [base] = 0 m. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the.. Chemistry Buffer Lab Report.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Chemistry Buffer Lab Report compare the results of the ph sensitivity data in parts a and c. Calculate the individual concentrations and. Solutions by utilizing a ph electrode. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Explain why the buffer capacity depends on the concentration of. Designing and preparing a buffer. • investigate the nature. Chemistry Buffer Lab Report.
From www.studocu.com
Experiment 4 Buffer Lab Report Chemistry 241L (Sections Studocu Chemistry Buffer Lab Report compare the results of the ph sensitivity data in parts a and c. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Designing and preparing a buffer. • investigate. Chemistry Buffer Lab Report.
From www.studocu.com
Report 2 p H AND Buffers lab 2 CHEMISTRY LABORATORY REPORT Chemistry Buffer Lab Report a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. compare the results of the ph sensitivity data in parts a and c. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. buffers are most effective. Chemistry Buffer Lab Report.
From www.studocu.com
Buffer lab report Lab 5 Efficacy of Buffers A Lab to Determine the Chemistry Buffer Lab Report Explain why the buffer capacity depends on the concentration of. [acid] + [base] = 0 m. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. compare the results of the ph sensitivity data in parts a and c. a buffer solution consists of a conjugate acid/base pair, so it. Chemistry Buffer Lab Report.
From fyojkjzvi.blob.core.windows.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Lab Report at Martin Chemistry Buffer Lab Report [acid] + [base] = 0 m. Explain why the buffer capacity depends on the concentration of. The purpose of this experiment is to test a buffer’s behavior and capacity with different. show calculations for the assigned buffer. compare the results of the ph sensitivity data in parts a and c. The dissociation of a weak acid in water. Chemistry Buffer Lab Report.
From www.studocu.com
Experiment 2 MBIO 2360 Lab Report 2 Experiment BUFFERS, BUFFER Chemistry Buffer Lab Report Explain why the buffer capacity depends on the concentration of. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. [acid] + [base] = 0 m. a buffer solution consists of. Chemistry Buffer Lab Report.
From www.studocu.com
CHEM 271 Lab Report 2 Experiment Buffers & Biochemical Analysis Chemistry Buffer Lab Report Designing and preparing a buffer. [acid] + [base] = 0 m. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Explain why the buffer capacity depends on the concentration of. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. show calculations for. Chemistry Buffer Lab Report.
From exyxywimb.blob.core.windows.net
Acid Base Buffer Lab Report at Douglas Foss blog Chemistry Buffer Lab Report [acid] + [base] = 0 m. Designing and preparing a buffer. Calculate the individual concentrations and. The purpose of this experiment is to test a buffer’s behavior and capacity with different. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. • investigate the nature of buffer solutions with. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 14 chem Acid, Bases, Salts and Buffers A 11/14/ F. Chem 1060013 Chemistry Buffer Lab Report Calculate the individual concentrations and. [acid] + [base] = 0 m. The purpose of this experiment is to test a buffer’s behavior and capacity with different. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. show calculations for the assigned buffer. compare the results. Chemistry Buffer Lab Report.
From studylib.net
Buffer Lab Chemistry Buffer Lab Report a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Explain why the buffer capacity depends on the concentration of. show calculations for the. Chemistry Buffer Lab Report.
From www.studocu.com
Experiment 13 A buffer is an aqueous solution that consist of a weak Chemistry Buffer Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Explain why the buffer capacity depends on the concentration of. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. The dissociation of a weak. Chemistry Buffer Lab Report.
From www.studocu.com
Working with buffers Lab Report BRAYAM CARDONA TA MARIO VENDRELL Chemistry Buffer Lab Report a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. show calculations for the assigned buffer. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. compare the results of the ph sensitivity data in. Chemistry Buffer Lab Report.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Chemistry Buffer Lab Report Designing and preparing a buffer. [acid] + [base] = 0 m. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small. Calculate the individual concentrations and. Explain why the buffer capacity depends on the concentration of. • investigate the nature of buffer solutions with respect to. Chemistry Buffer Lab Report.
From www.studocu.com
Report 2 CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND BUFFERS Chemistry Buffer Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. a buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition. Chemistry Buffer Lab Report.
From www.studocu.com
Chem Lab Report 2 Guideline CHEMISTRY LABORATORY REPORT EXPERIMENT Chemistry Buffer Lab Report show calculations for the assigned buffer. The purpose of this experiment is to test a buffer’s behavior and capacity with different. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. compare the results of the ph sensitivity data in parts a and c. Solutions by utilizing a. Chemistry Buffer Lab Report.
From www.chegg.com
Buffer lab report ? Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong. Designing and preparing a buffer. a buffer solution consists of a conjugate acid/base pair, so it maintains. Chemistry Buffer Lab Report.
From www.studocu.com
Report Expt GENERAL CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND Chemistry Buffer Lab Report Solutions by utilizing a ph electrode. buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. [acid] + [base] = 0 m. Designing and preparing a buffer. compare the results of the ph sensitivity data in parts a and c. • investigate the nature of buffer solutions with. Chemistry Buffer Lab Report.
From www.studocu.com
Chemlab report 2 CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Solutions by utilizing a ph electrode. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer. Chemistry Buffer Lab Report.
From www.studocu.com
Lab 5 chem 105b buffers lab solutions Eva Hilton Chem 105b Lab Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. compare the results of the ph sensitivity data in parts a and c. show calculations for the assigned buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Designing. Chemistry Buffer Lab Report.
From www.studocu.com
Chemistry II Lab Report Buffers and Common Ion Effects Buffers and Chemistry Buffer Lab Report buffers are most effective at protecting against ph change when they have similar concentrations of both components of the. compare the results of the ph sensitivity data in parts a and c. The purpose of this experiment is to test a buffer’s behavior and capacity with different. Designing and preparing a buffer. show calculations for the assigned. Chemistry Buffer Lab Report.