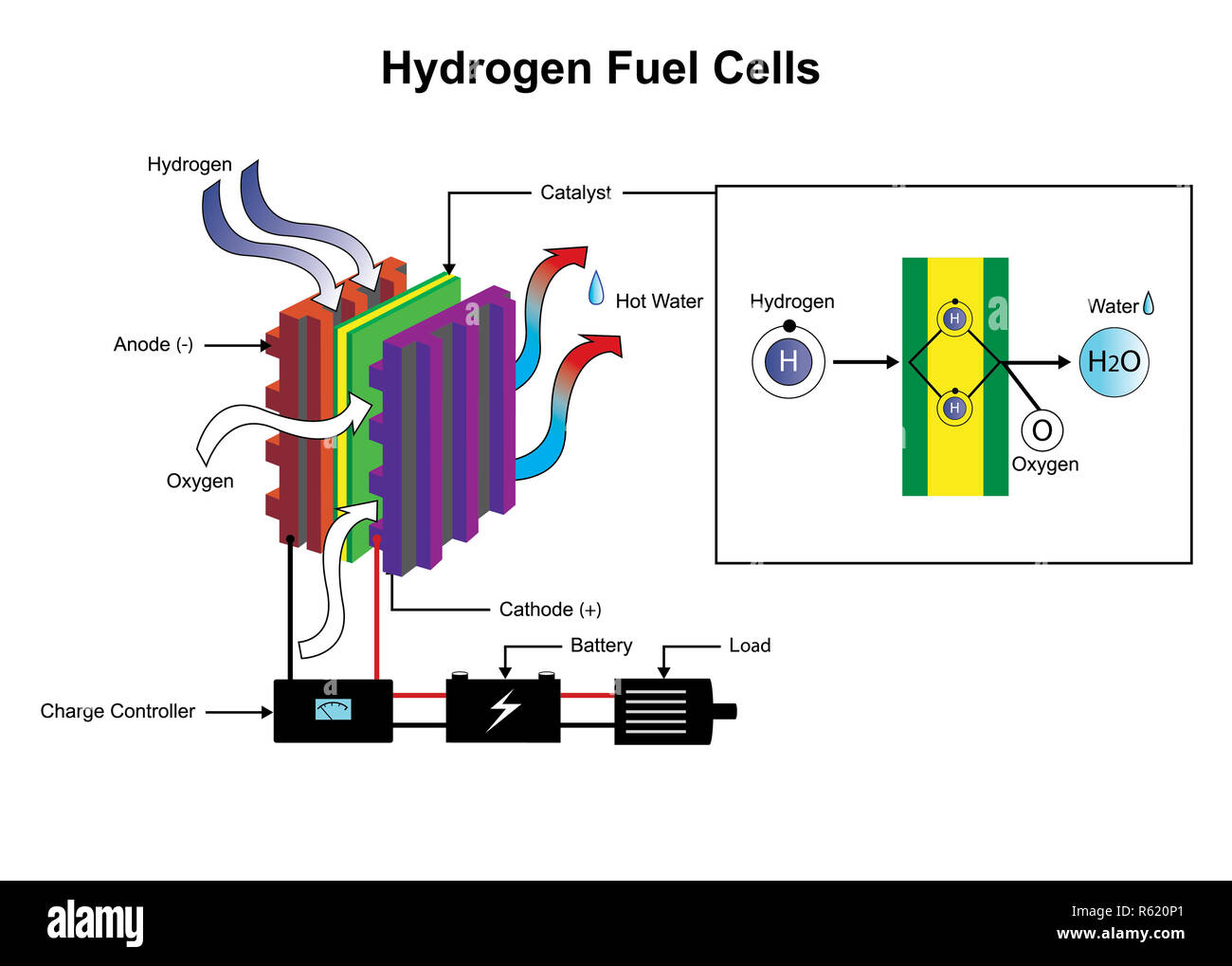
Examples Of Fuel Cell In Chemistry . Fuel cells are similar to. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. A fuel cell is a device that converts chemical energy into electrical energy. fuel cells are classified primarily by the kind of electrolyte they employ. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol directly as a. It’s like a battery, but. This classification determines the kind of. A chemical cell produces a voltage until one of the reactants is used up. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water.
from www.alamy.com
A fuel cell is a device that converts chemical energy into electrical energy. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol directly as a. It’s like a battery, but. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This classification determines the kind of. fuel cells are classified primarily by the kind of electrolyte they employ. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. Fuel cells are similar to. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. A chemical cell produces a voltage until one of the reactants is used up.
Diagram of fuel cell hires stock photography and images Alamy
Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. A fuel cell is a device that converts chemical energy into electrical energy. It’s like a battery, but. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. This classification determines the kind of. Fuel cells are similar to. fuel cells are classified primarily by the kind of electrolyte they employ. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol directly as a. A chemical cell produces a voltage until one of the reactants is used up.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Examples Of Fuel Cell In Chemistry It’s like a battery, but. fuel cells are classified primarily by the kind of electrolyte they employ. This classification determines the kind of. Fuel cells are similar to. A chemical cell produces a voltage until one of the reactants is used up. fuel cell, any of a class of devices that convert the chemical energy of a fuel. Examples Of Fuel Cell In Chemistry.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Examples Of Fuel Cell In Chemistry fuel cells are classified primarily by the kind of electrolyte they employ. A chemical cell produces a voltage until one of the reactants is used up. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. This classification determines the kind of. fuel. Examples Of Fuel Cell In Chemistry.
From www.youtube.com
Fuel Cells O Level IGCSE Chemistry YouTube Examples Of Fuel Cell In Chemistry This classification determines the kind of. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is a device that converts chemical energy into electrical energy. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The direct. Examples Of Fuel Cell In Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Examples Of Fuel Cell In Chemistry a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol directly as a. Fuel cells are similar to. A chemical cell produces a voltage until. Examples Of Fuel Cell In Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Examples Of Fuel Cell In Chemistry It’s like a battery, but. fuel cells are classified primarily by the kind of electrolyte they employ. Fuel cells are similar to. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity by using a chemical reaction. Examples Of Fuel Cell In Chemistry.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Examples Of Fuel Cell In Chemistry A fuel cell is a device that converts chemical energy into electrical energy. fuel cells are classified primarily by the kind of electrolyte they employ. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A chemical cell produces a voltage until one of. Examples Of Fuel Cell In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Examples Of Fuel Cell In Chemistry It’s like a battery, but. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. A chemical cell produces a voltage until one of the reactants is used up. This classification determines the kind of. Fuel cells are similar to. A fuel cell is a. Examples Of Fuel Cell In Chemistry.
From www.dreamstime.com
The Alkaline Fuel Cell Structure. Stock Vector Illustration of flow Examples Of Fuel Cell In Chemistry fuel cells are classified primarily by the kind of electrolyte they employ. Fuel cells are similar to. This classification determines the kind of. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a device that converts chemical energy into. Examples Of Fuel Cell In Chemistry.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Examples Of Fuel Cell In Chemistry It’s like a battery, but. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells are classified. Examples Of Fuel Cell In Chemistry.
From siqens.de
Principle, types, advantages What is a fuel cell? SIQENS Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. Fuel cells are similar to. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. a fuel cell like this will continue. Examples Of Fuel Cell In Chemistry.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Examples Of Fuel Cell In Chemistry a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. This classification determines the kind of. Fuel cells are similar to. fuel. Examples Of Fuel Cell In Chemistry.
From chem.libretexts.org
20.7 Batteries and Fuel Cells Chemistry LibreTexts Examples Of Fuel Cell In Chemistry A chemical cell produces a voltage until one of the reactants is used up. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and. Examples Of Fuel Cell In Chemistry.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture Examples Of Fuel Cell In Chemistry A fuel cell is a device that converts chemical energy into electrical energy. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This classification determines the kind of. a fuel cell is a device that generates electricity by using a chemical reaction to. Examples Of Fuel Cell In Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Examples Of Fuel Cell In Chemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are similar to. fuel cells are classified primarily by the kind of electrolyte they employ. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell. Examples Of Fuel Cell In Chemistry.
From schematiclistpact101.z22.web.core.windows.net
Diagram Fuel Cell Examples Of Fuel Cell In Chemistry It’s like a battery, but. A chemical cell produces a voltage until one of the reactants is used up. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell is a device that generates electricity by using a chemical reaction to. Examples Of Fuel Cell In Chemistry.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Examples Of Fuel Cell In Chemistry A fuel cell is a device that converts chemical energy into electrical energy. A chemical cell produces a voltage until one of the reactants is used up. It’s like a battery, but. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells. Examples Of Fuel Cell In Chemistry.
From www.youtube.com
Proton Exchange Membrane Fuel Cell, Introduction, Principle, Advantages Examples Of Fuel Cell In Chemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. It’s like a battery, but. fuel cells are classified primarily by the kind of electrolyte they employ. Fuel cells are similar to. A chemical cell produces a voltage until one of the reactants is. Examples Of Fuel Cell In Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. It’s like a battery, but. A fuel cell is a device that converts chemical energy into electrical energy. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol. Examples Of Fuel Cell In Chemistry.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Examples Of Fuel Cell In Chemistry A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is a device that converts chemical energy into electrical energy. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. a fuel cell like this will continue. Examples Of Fuel Cell In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Examples Of Fuel Cell In Chemistry a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. It’s like a battery, but. A fuel cell is a device that converts. Examples Of Fuel Cell In Chemistry.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. It’s like a battery, but. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The direct methanol fuel cells (dmfc) this type. Examples Of Fuel Cell In Chemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Examples Of Fuel Cell In Chemistry A chemical cell produces a voltage until one of the reactants is used up. fuel cells are classified primarily by the kind of electrolyte they employ. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. The direct methanol fuel cells (dmfc) this type. Examples Of Fuel Cell In Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. This classification determines the kind of. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells are similar to. fuel cells are classified primarily by the kind of electrolyte they employ.. Examples Of Fuel Cell In Chemistry.
From saylordotorg.github.io
Applications of Redox Reactions Voltaic Cells Examples Of Fuel Cell In Chemistry Fuel cells are similar to. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells are classified primarily by the kind of electrolyte they employ. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology. Examples Of Fuel Cell In Chemistry.
From www.simplechemconcepts.com
Hydrogen Oxygen Fuel Cells in O Level and IP Pure Chemistry Examples Of Fuel Cell In Chemistry fuel cells are classified primarily by the kind of electrolyte they employ. Fuel cells are similar to. A chemical cell produces a voltage until one of the reactants is used up. This classification determines the kind of. A fuel cell is a device that converts chemical energy into electrical energy. a fuel cell like this will continue to. Examples Of Fuel Cell In Chemistry.
From www.shalom-education.com
Fuel Cells GCSE Chemistry Revision Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A chemical cell produces a voltage until one of the reactants is used. Examples Of Fuel Cell In Chemistry.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Examples Of Fuel Cell In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. It’s like a battery, but. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. Fuel cells are similar to. fuel cells. Examples Of Fuel Cell In Chemistry.
From www.researchgate.net
10 Fuel cell chemical reaction [4] Download Scientific Diagram Examples Of Fuel Cell In Chemistry This classification determines the kind of. Fuel cells are similar to. It’s like a battery, but. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer technology but uses methanol directly as a. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen. Examples Of Fuel Cell In Chemistry.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Examples Of Fuel Cell In Chemistry A fuel cell is a device that converts chemical energy into electrical energy. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells are similar to. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. The direct methanol fuel cells (dmfc). Examples Of Fuel Cell In Chemistry.
From www.alamy.com
Diagram of fuel cell hires stock photography and images Alamy Examples Of Fuel Cell In Chemistry A chemical cell produces a voltage until one of the reactants is used up. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. Fuel cells are similar to. This classification determines the kind of. A fuel cell is a device that converts chemical energy into electrical energy.. Examples Of Fuel Cell In Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Examples Of Fuel Cell In Chemistry a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. A chemical cell produces a voltage until one of the reactants is used up. It’s like a battery, but. This classification determines the kind of. Fuel cells are similar to. fuel cell, any of. Examples Of Fuel Cell In Chemistry.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Examples Of Fuel Cell In Chemistry It’s like a battery, but. A fuel cell is a device that converts chemical energy into electrical energy. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of. Examples Of Fuel Cell In Chemistry.
From www.collidu.com
Fuel Cell PowerPoint Presentation Slides PPT Template Examples Of Fuel Cell In Chemistry A chemical cell produces a voltage until one of the reactants is used up. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. Fuel cells are similar to. The direct methanol fuel cells (dmfc) this type of fuel cell is based on solid polymer. Examples Of Fuel Cell In Chemistry.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Examples Of Fuel Cell In Chemistry a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. A fuel cell is a device that converts chemical energy into electrical energy. It’s like a battery, but. This classification determines the kind of. fuel cells are classified primarily by the kind of electrolyte. Examples Of Fuel Cell In Chemistry.
From www.slideserve.com
PPT Fuel Cell PowerPoint Presentation, free download ID5891466 Examples Of Fuel Cell In Chemistry fuel cells are classified primarily by the kind of electrolyte they employ. A chemical cell produces a voltage until one of the reactants is used up. It’s like a battery, but. a fuel cell is a device that generates electricity by using a chemical reaction to convert fuel and oxygen into electricity, heat, and water. The direct methanol. Examples Of Fuel Cell In Chemistry.