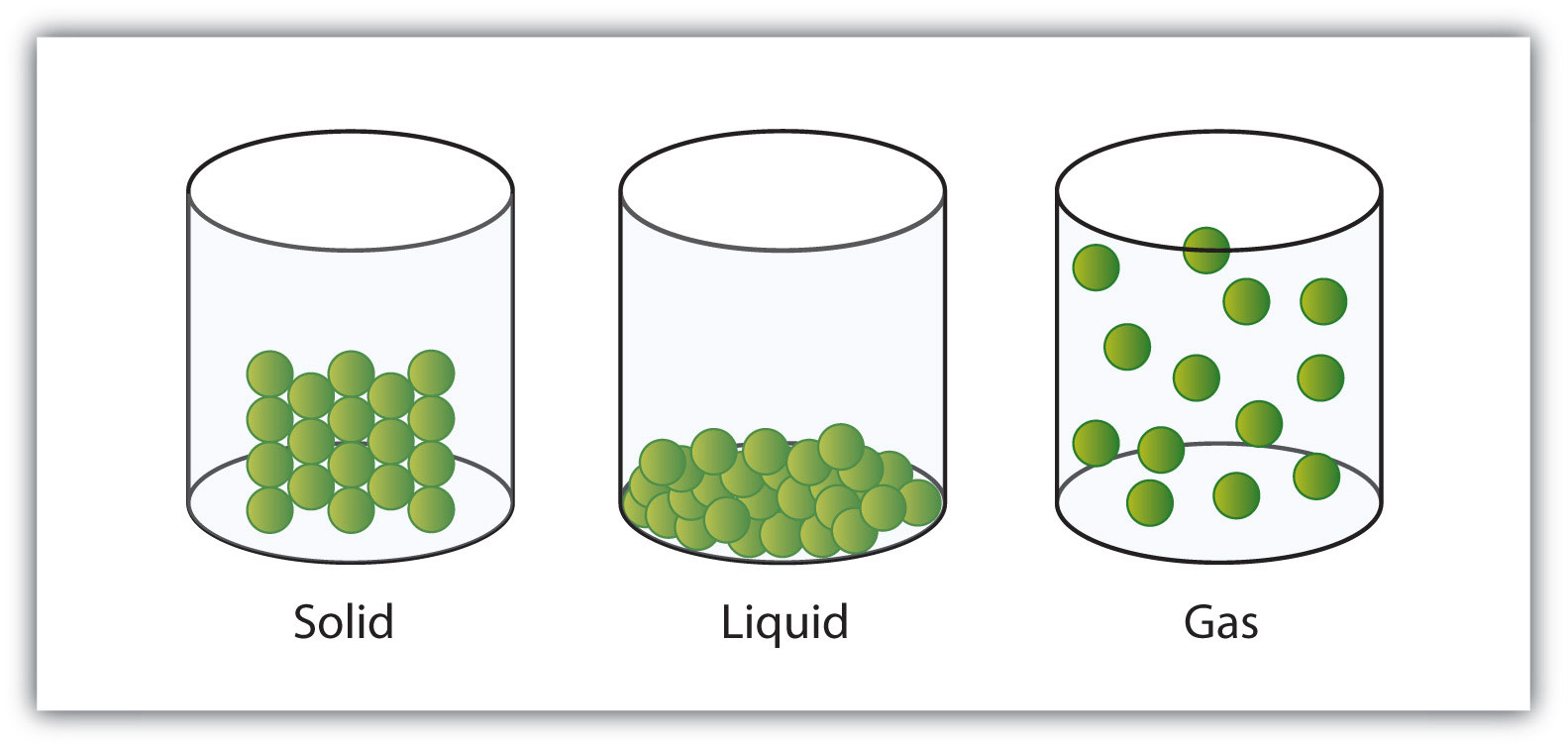
Does A Molecule Have Volume . The molar volume can be calculated in substances in. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Solids have a definite shape and volume. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. At stp, one mole (6.02 × 1023 representative particles) of. In a liquid, the particles are still in close contact, so liquids have a definite volume. Liquids have a definite volume, but take the shape of the container. This describes the liquid state. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The molar volume of a gas is the volume of one mole of a gas at stp.
from saylordotorg.github.io
At stp, one mole (6.02 × 1023 representative particles) of. In a liquid, the particles are still in close contact, so liquids have a definite volume. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). This describes the liquid state. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. Liquids have a definite volume, but take the shape of the container. The molar volume can be calculated in substances in. The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. Solids have a definite shape and volume.
Solids and Liquids
Does A Molecule Have Volume The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. At stp, one mole (6.02 × 1023 representative particles) of. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The molar volume can be calculated in substances in. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Solids have a definite shape and volume. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. The molar volume of a gas is the volume of one mole of a gas at stp. Liquids have a definite volume, but take the shape of the container. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. This describes the liquid state. In a liquid, the particles are still in close contact, so liquids have a definite volume.
From www.britannica.com
molecule Definition, Examples, Structures, & Facts Britannica Does A Molecule Have Volume The molar volume can be calculated in substances in. In a liquid, the particles are still in close contact, so liquids have a definite volume. Liquids have a definite volume, but take the shape of the container. Solids have a definite shape and volume. This describes the liquid state. If the particles of a substance have enough energy to partially. Does A Molecule Have Volume.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. Liquids have a definite volume, but take the shape of the container. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. This describes the liquid state. The volume (\ (v\)) of. Does A Molecule Have Volume.
From www.worksheetsplanet.com
What is a Molecule Meaning & Definition Does A Molecule Have Volume The molar volume can be calculated in substances in. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. Liquids have a definite volume, but take the shape of the container. Solids have a definite shape and volume. In a liquid, the particles are. Does A Molecule Have Volume.
From www.vecteezy.com
Chemistry model molecule carbon dioxide CO2 scientific element formula. Integrated particles Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. In a liquid, the particles are still in close contact, so liquids have a definite volume.. Does A Molecule Have Volume.
From www.yourdictionary.com
Basic Difference Between an Atom and a Molecule YourDictionary Does A Molecule Have Volume At stp, one mole (6.02 × 1023 representative particles) of. The molar volume of a gas is the volume of one mole of a gas at stp. Liquids have a definite volume, but take the shape of the container. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the. Does A Molecule Have Volume.
From haikudeck.com
Science by Rylan Meier Does A Molecule Have Volume If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. In a liquid, the particles are still in close contact, so liquids have a definite volume. This describes the liquid state. Liquids have a definite volume, but take the shape of the container. The. Does A Molecule Have Volume.
From sciencenotes.org
Volume Definition in Science Does A Molecule Have Volume The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The molar volume can be calculated in substances in. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. This describes the. Does A Molecule Have Volume.
From www.researchgate.net
Visualization of a large molecule. Surface and volume rendering... Download Scientific Diagram Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. Liquids have a definite volume, but take the shape of the container. This describes the liquid state. The molar volume can be calculated in substances in. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles. Does A Molecule Have Volume.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube Does A Molecule Have Volume This describes the liquid state. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. In a liquid, the particles are still in close contact, so liquids have a definite volume. The molar volume of a gas is the volume of one mole of a gas at stp. The. Does A Molecule Have Volume.
From www.sciencefacts.net
Molecule Definition, Examples, Facts & Diagram Does A Molecule Have Volume If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. The molar volume can be calculated in substances in. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the. Does A Molecule Have Volume.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement and energy of the Does A Molecule Have Volume At stp, one mole (6.02 × 1023 representative particles) of. The molar volume can be calculated in substances in. Liquids have a definite volume, but take the shape of the container. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. Solids have a. Does A Molecule Have Volume.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Does A Molecule Have Volume The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). In a liquid, the particles are still in close contact,. Does A Molecule Have Volume.
From worksheetlisteb.z13.web.core.windows.net
Picture Of Solid Liquid And Gas Does A Molecule Have Volume Liquids have a definite volume, but take the shape of the container. The molar volume of a gas is the volume of one mole of a gas at stp. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. At stp, one mole (6.02. Does A Molecule Have Volume.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Does A Molecule Have Volume The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). This describes the liquid state. Liquids have a definite volume, but take the shape of the container. If the particles of a substance have enough energy to partially overcome intermolecular interactions,. Does A Molecule Have Volume.
From www.chegg.com
Solved P2. A triatomic molecule can have a linear Does A Molecule Have Volume The molar volume can be calculated in substances in. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature. Does A Molecule Have Volume.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Does A Molecule Have Volume Liquids have a definite volume, but take the shape of the container. At stp, one mole (6.02 × 1023 representative particles) of. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Solids have a definite shape and volume. This describes. Does A Molecule Have Volume.
From www.showme.com
Calculating the number of molecules from moles oxygen Chemistry, Moles, Unit Conversion ShowMe Does A Molecule Have Volume Solids have a definite shape and volume. Liquids have a definite volume, but take the shape of the container. In a liquid, the particles are still in close contact, so liquids have a definite volume. The molar volume can be calculated in substances in. This describes the liquid state. The volume (\ (v\)) of an ideal gas varies directly with. Does A Molecule Have Volume.
From sciencenotes.org
What Is a Molecule? Definition and Examples Does A Molecule Have Volume The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. This describes the liquid state. Liquids have a definite volume, but take. Does A Molecule Have Volume.
From www.chemistry-teaching-resources.com
chemistry picture Does A Molecule Have Volume Solids have a definite shape and volume. Liquids have a definite volume, but take the shape of the container. In a liquid, the particles are still in close contact, so liquids have a definite volume. This describes the liquid state. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about. Does A Molecule Have Volume.
From ecampusontario.pressbooks.pub
9.5 The Theory Chemistry Does A Molecule Have Volume Liquids have a definite volume, but take the shape of the container. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. Solids have a definite shape and volume. The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume,. Does A Molecule Have Volume.
From www.vecteezy.com
Chemistry model molecule water H2O scientific element formula. Integrated particles natural Does A Molecule Have Volume Liquids have a definite volume, but take the shape of the container. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. In a liquid, the particles are still in close contact, so liquids have a definite volume. Solids have a definite shape and. Does A Molecule Have Volume.
From saylordotorg.github.io
Solids and Liquids Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. Solids have a definite shape and volume. This describes the liquid state. In a liquid, the particles are still in close contact, so liquids have a definite volume. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c. Does A Molecule Have Volume.
From www.researchgate.net
Diffusion volumes of some atom and molecules Download Scientific Diagram Does A Molecule Have Volume At stp, one mole (6.02 × 1023 representative particles) of. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. The molar volume. Does A Molecule Have Volume.
From ar.inspiredpencil.com
Volume Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. In a liquid, the particles are still in close contact, so liquids have a definite volume. At stp, one mole (6.02 × 1023 representative particles) of. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the. Does A Molecule Have Volume.
From www.pinterest.com
How Much Is a Mole of Water? Mass and Volume Gcse Chemistry, Chemistry Notes, Chemistry Lessons Does A Molecule Have Volume The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. The molar volume can be calculated in substances in. Solids have a definite shape and volume. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p). Does A Molecule Have Volume.
From www.pinterest.com
Pin on School Does A Molecule Have Volume If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. Liquids have a definite volume, but take the shape of the container. At stp, one mole (6.02 × 1023 representative particles) of. The volume (\ (v\)) of an ideal gas varies directly with the. Does A Molecule Have Volume.
From courses.lumenlearning.com
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Chemistry Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. At stp, one mole (6.02 × 1023 representative particles) of. Liquids have a definite volume, but. Does A Molecule Have Volume.
From slideplayer.com
The Mole Concept. ppt download Does A Molecule Have Volume At stp, one mole (6.02 × 1023 representative particles) of. The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. This describes the liquid state. The. Does A Molecule Have Volume.
From study.com
Simple Molecules Examples & Explanation Video & Lesson Transcript Does A Molecule Have Volume Solids have a definite shape and volume. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. At stp, one mole (6.02 ×. Does A Molecule Have Volume.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Does A Molecule Have Volume The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. The volume (\ (v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). In a liquid, the. Does A Molecule Have Volume.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Does A Molecule Have Volume Solids have a definite shape and volume. At stp, one mole (6.02 × 1023 representative particles) of. Liquids have a definite volume, but take the shape of the container. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. This describes the liquid state. If the particles of a. Does A Molecule Have Volume.
From www.sliderbase.com
Elements Presentation Chemistry Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. Solids have a definite shape and volume. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. The molar volume of an ideal gas under normal. Does A Molecule Have Volume.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core Concepts in Chemistry Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume can be calculated in substances in. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact. This describes the liquid state. The molar volume. Does A Molecule Have Volume.
From www.vecteezy.com
Chemistry model molecule ozone O3 gas scientific element formula. Integrated particles natural Does A Molecule Have Volume Liquids have a definite volume, but take the shape of the container. Solids have a definite shape and volume. The molar volume, symbol $v_\mathrm m$ is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. This describes the liquid state. At stp, one mole (6.02 × 1023 representative particles). Does A Molecule Have Volume.
From www.slideserve.com
PPT Molar Volume PowerPoint Presentation ID5715901 Does A Molecule Have Volume The molar volume of a gas is the volume of one mole of a gas at stp. The molar volume can be calculated in substances in. Solids have a definite shape and volume. The molar volume of an ideal gas under normal conditions (1 atmosphere pressure and 0°c temperature) is equal to 22.4 liters/mol. This describes the liquid state. At. Does A Molecule Have Volume.