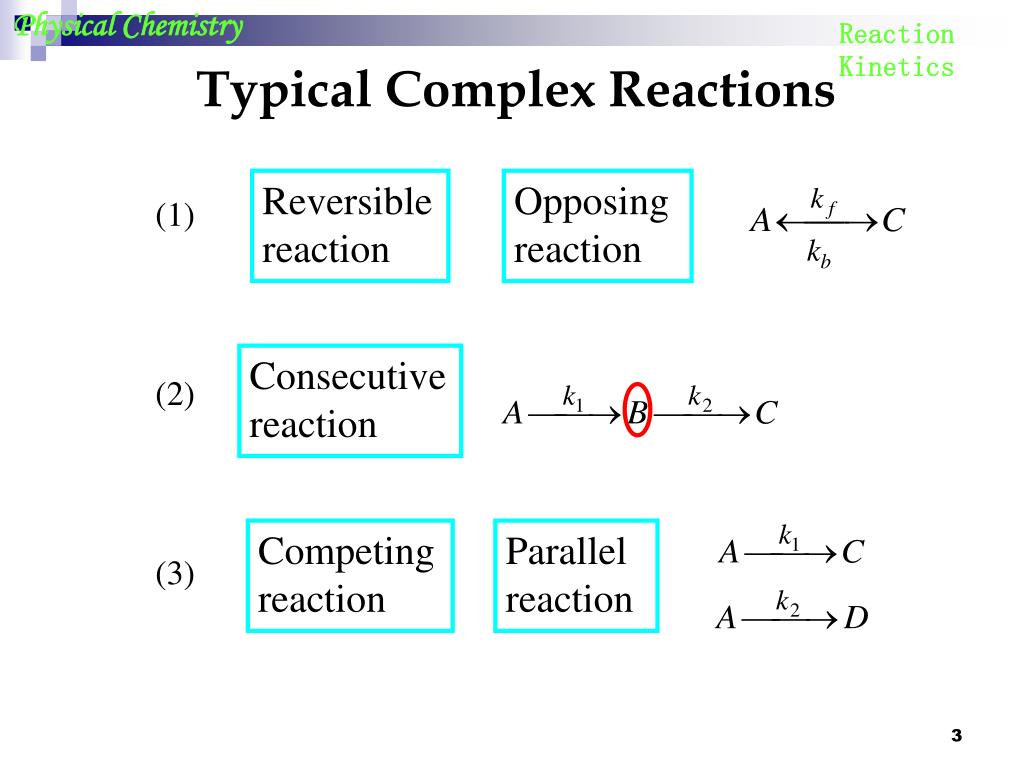
Chemical Kinetics Reversible Reactions . This chapter describes the most common cases for reversible. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Analysis of the sequence of. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Reversible reactions of first order have the stoichiometry and kinetic model. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Consider the following reversible reaction in which the reaction is first order in both directions: There are different flavors that can be implement depending on the. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution.
from www.slideserve.com
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Consider the following reversible reaction in which the reaction is first order in both directions: Analysis of the sequence of. There are different flavors that can be implement depending on the. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Reversible reactions of first order have the stoichiometry and kinetic model. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: This chapter describes the most common cases for reversible. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution.
PPT Reaction (2) PowerPoint Presentation, free download ID
Chemical Kinetics Reversible Reactions A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. There are different flavors that can be implement depending on the. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: This chapter describes the most common cases for reversible. Consider the following reversible reaction in which the reaction is first order in both directions: Reversible reactions of first order have the stoichiometry and kinetic model. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Analysis of the sequence of.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID4983216 Chemical Kinetics Reversible Reactions In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Analysis of the sequence of. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Study into the kinetics of a chemical reaction is. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Chapter 13 Lecture 1 Chemical PowerPoint Presentation Chemical Kinetics Reversible Reactions Reversible reactions of first order have the stoichiometry and kinetic model. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Consider the following reversible reaction in which the reaction is first order in both directions: In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. This chapter describes the most common cases for reversible. Study. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction (2) PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. There are different flavors that can be implement depending on the. A reversible reaction is a reaction in which the conversion of reactants to. Chemical Kinetics Reversible Reactions.
From twig.sg
Reaction Courses Chemical Kinetics Reversible Reactions There are different flavors that can be implement depending on the. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Reversible reactions of first order have the stoichiometry and kinetic model. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions. Chemical Kinetics Reversible Reactions.
From www.slideshare.net
Chapter 14 Lecture Chemical Chemical Kinetics Reversible Reactions Consider the following reversible reaction in which the reaction is first order in both directions: This chapter describes the most common cases for reversible. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution.. Chemical Kinetics Reversible Reactions.
From www.youtube.com
Chemical Parallel Reactions Competitive Reaction Solved Chemical Kinetics Reversible Reactions Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: This chapter describes the most common cases for reversible. Reversible reactions of first order have the stoichiometry and kinetic model.. Chemical Kinetics Reversible Reactions.
From www.youtube.com
Chemical Introduction (with Animation) YouTube Chemical Kinetics Reversible Reactions Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Analysis of the sequence of. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. This chapter describes the most common cases for reversible. There are different flavors that can be implement depending on the. A reversible reaction is a reaction in which the conversion of. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction (2) PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions Reversible reactions of first order have the stoichiometry and kinetic model. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. A reversible reaction is a reaction in which the conversion of reactants to. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4567208 Chemical Kinetics Reversible Reactions Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Reversible reactions of first order have the stoichiometry and kinetic model. There are different flavors that can be implement depending on the. Analysis of the sequence of. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. A reversible reaction is a reaction in which the. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Analysis of the sequence of. There are different flavors that can be implement depending on the. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Consider the following reversible. Chemical Kinetics Reversible Reactions.
From www.youtube.com
Chemical 04 Reversible Reactions For Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Analysis of the sequence of. Consider the following reversible reaction in which the reaction is first order in both directions: This chapter describes the most common cases for reversible. Reversible reactions of first order have the. Chemical Kinetics Reversible Reactions.
From www.bartleby.com
Chemical bartleby Chemical Kinetics Reversible Reactions Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: Analysis of the sequence of. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Reversible reactions of first order have the stoichiometry and kinetic model. There are different flavors. Chemical Kinetics Reversible Reactions.
From studylib.net
CHEMICAL Chemical Kinetics Reversible Reactions Analysis of the sequence of. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. This chapter describes the most common cases for reversible. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. There are different flavors that can. Chemical Kinetics Reversible Reactions.
From studylib.net
2. Chemical Chemical Kinetics Reversible Reactions In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Analysis of the sequence of. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products. Chemical Kinetics Reversible Reactions.
From www.pinterest.ph
Difference between Reversible reaction and Irreversible reaction Chemical Kinetics Reversible Reactions There are different flavors that can be implement depending on the. Analysis of the sequence of. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: Consider the following reversible. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Chapter X of Complex Reactions PowerPoint Presentation Chemical Kinetics Reversible Reactions A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. There are different flavors that can be implement depending on the. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Reversible reactions of. Chemical Kinetics Reversible Reactions.
From slideplayer.com
Reversible Reactions and Equilbrium ppt download Chemical Kinetics Reversible Reactions Analysis of the sequence of. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Study into the kinetics of a chemical reaction is. Chemical Kinetics Reversible Reactions.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Chemical Kinetics Reversible Reactions Analysis of the sequence of. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. There are different flavors that can be implement depending on the. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. This chapter describes the most common cases for reversible. Reversible reactions of first order have the stoichiometry and kinetic model.. Chemical Kinetics Reversible Reactions.
From www.youtube.com
Rate law of reversible reaction Chemical YouTube Chemical Kinetics Reversible Reactions Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: Reversible reactions of first order have the stoichiometry and kinetic model. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. There are different flavors that can be implement depending. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Chemical Khadijah Hanim Abdul Rahman UniMAP PowerPoint Chemical Kinetics Reversible Reactions A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Analysis of the sequence of. This chapter describes the most common cases for reversible. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution.. Chemical Kinetics Reversible Reactions.
From www.sciencemotive.com
HalfLife of Reactions in Chemical ScienceMotive Chemical Kinetics Reversible Reactions Reversible reactions of first order have the stoichiometry and kinetic model. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Analysis of the sequence of. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Study into the kinetics of a chemical reaction is usually carried out with one or both. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT 15 Chemical PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions There are different flavors that can be implement depending on the. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Analysis of the sequence of. Reversible reactions of first. Chemical Kinetics Reversible Reactions.
From slideplayer.com
Reversible Reactions and Equilibrium ppt download Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. There are different flavors that can be implement depending on the. Analysis of the sequence of. This chapter describes the most common cases for reversible. Consider the following reversible reaction in which the reaction is first order in both directions: Reversible reactions of first order have the stoichiometry and kinetic model. A reversible reaction. Chemical Kinetics Reversible Reactions.
From slideplayer.com
Chemical and the Nucleus, a Chemist’s View ppt download Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. There are different flavors that can be implement depending on the. Reversible reactions of first order have the stoichiometry and kinetic model. This chapter describes the most common cases for reversible. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Study. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction 1 st order reactions PowerPoint Presentation Chemical Kinetics Reversible Reactions A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. This chapter describes the most common cases for reversible. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Analysis of the sequence of. Consider the following reversible reaction in. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Aquatic Chemical PowerPoint Presentation, free download Chemical Kinetics Reversible Reactions Reversible reactions of first order have the stoichiometry and kinetic model. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Consider the following reversible reaction in which the reaction is first order in both directions: Analysis of the sequence of. There are different flavors that can be. Chemical Kinetics Reversible Reactions.
From ppt-online.org
Chemical презентация онлайн Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Consider the following reversible reaction in which the reaction is first order in both directions: A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants. Chemical Kinetics Reversible Reactions.
From revisechemistry.uk
Reversible Reactions and Dynamic Equilibrium AQA C6 revisechemistry.uk Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. Reversible reactions of first order have the stoichiometry and kinetic model. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: A reversible reaction. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2089937 Chemical Kinetics Reversible Reactions Analysis of the sequence of. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. There are different flavors that can be implement depending on the. This chapter describes the most common cases for reversible. $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. In the following sections, we will derive. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction (2) PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. There are different flavors that can be implement depending on the. This chapter describes the most common cases for reversible. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive.. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction (2) PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions Flow instruments are a rapid mixing devices used to study the chemical kinetics of fast reactions in solution. There are different flavors that can be implement depending on the. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Reversible reactions of first order have the stoichiometry and kinetic model. Study into. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID1156612 Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: There are different flavors that can be implement depending on. Chemical Kinetics Reversible Reactions.
From ppt-online.org
Chemical презентация онлайн Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. Analysis of the sequence of. Consider the following reversible reaction in which the reaction is first order in both directions: There are different flavors that can be implement depending on the. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Flow instruments are a rapid. Chemical Kinetics Reversible Reactions.
From www-jmg.ch.cam.ac.uk
notes Chemical Kinetics Reversible Reactions There are different flavors that can be implement depending on the. Consider the following reversible reaction in which the reaction is first order in both directions: Analysis of the sequence of. In the following sections, we will derive rate laws for complex reaction mechanisms, including reversible, parallel and consecutive. Flow instruments are a rapid mixing devices used to study the. Chemical Kinetics Reversible Reactions.
From www.slideserve.com
PPT Reaction (2) PowerPoint Presentation, free download ID Chemical Kinetics Reversible Reactions $$\ce{[a] <=> [b]}$$ $k_\mathrm a$ is. This chapter describes the most common cases for reversible. Analysis of the sequence of. Study into the kinetics of a chemical reaction is usually carried out with one or both of two main goals in mind: There are different flavors that can be implement depending on the. Reversible reactions of first order have the. Chemical Kinetics Reversible Reactions.