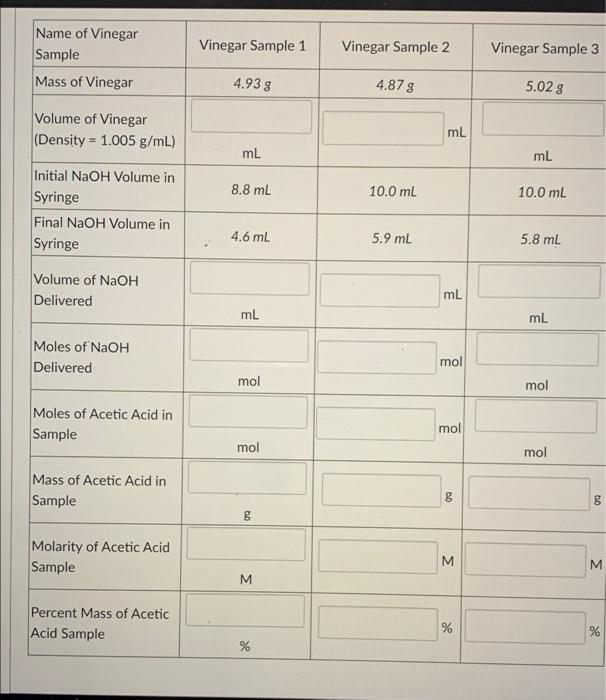
Vinegar Questionnaire Sample . (2) to use the standardized sodium hydroxide solution. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. To determine the percent by mass of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Calculate the volume of vinegar. A few drops will indicate the endpoint of the titration (color. A titration setup is used for the. A titration involves performing a controlled reaction between a solution of. Weigh each flask with the same balance and record the masses in your notebook. For precise measurement of the vinegar sample. (1) to standardize a solution of sodium hydroxide;
from www.chegg.com
To determine the percent by mass of acetic acid in vinegar. For precise measurement of the vinegar sample. Calculate the volume of vinegar. Weigh each flask with the same balance and record the masses in your notebook. A titration involves performing a controlled reaction between a solution of. A few drops will indicate the endpoint of the titration (color. (1) to standardize a solution of sodium hydroxide; (2) to use the standardized sodium hydroxide solution. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar.
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3
Vinegar Questionnaire Sample To determine the percent by mass of acetic acid in vinegar. A few drops will indicate the endpoint of the titration (color. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. A titration setup is used for the. (2) to use the standardized sodium hydroxide solution. (1) to standardize a solution of sodium hydroxide; For precise measurement of the vinegar sample. A titration involves performing a controlled reaction between a solution of. Calculate the volume of vinegar. To determine the percent by mass of acetic acid in vinegar. Weigh each flask with the same balance and record the masses in your notebook.
From old.sermitsiaq.ag
Printable Survey Template Vinegar Questionnaire Sample For precise measurement of the vinegar sample. A titration involves performing a controlled reaction between a solution of. Weigh each flask with the same balance and record the masses in your notebook. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. (2) to use the standardized sodium hydroxide. Vinegar Questionnaire Sample.
From www.numerade.com
SOLVED can you explain the analysis of household vinegar lab in the Vinegar Questionnaire Sample Weigh each flask with the same balance and record the masses in your notebook. (1) to standardize a solution of sodium hydroxide; The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample.. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Dale Vinegar 1. Preparation of Vinegar Sample Vinegar Questionnaire Sample To determine the percent by mass of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. Weigh each flask with the same balance and record the masses in your notebook. Calculate the volume of vinegar. A few drops will indicate the endpoint of the titration (color. A titration. Vinegar Questionnaire Sample.
From www.numerade.com
SOLVED PHASE 4 Titrate vinegar sample Complete the following steps Vinegar Questionnaire Sample Weigh each flask with the same balance and record the masses in your notebook. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Calculate the volume of vinegar. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 Vinegar Questionnaire Sample For precise measurement of the vinegar sample. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Weigh each flask with the same balance and record the masses in your notebook. A titration setup is used for the. (2) to use the standardized sodium hydroxide solution. A titration involves. Vinegar Questionnaire Sample.
From www.studeersnel.nl
Questionnaire 3 final vragenlijst 3 Questionnaire Fish and chips Vinegar Questionnaire Sample (2) to use the standardized sodium hydroxide solution. A titration setup is used for the. (1) to standardize a solution of sodium hydroxide; For precise measurement of the vinegar sample. A titration involves performing a controlled reaction between a solution of. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 Vinegar Questionnaire Sample In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Calculate the volume of vinegar. (1) to standardize a solution of sodium hydroxide; A few drops will indicate the endpoint of the titration (color. Weigh each flask with the same balance and record the masses in your notebook. A. Vinegar Questionnaire Sample.
From www.studocu.com
vinegar analysis lab procedure 32 EXPERIMENT 4 Vinegar Analysis Vinegar Questionnaire Sample A titration involves performing a controlled reaction between a solution of. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. Weigh each flask with the same balance and record the masses in your notebook. (2) to use the standardized sodium hydroxide solution. For precise measurement of the vinegar sample. The purpose of. Vinegar Questionnaire Sample.
From www.studocu.com
Lab 3 Vinegar Analysis In lab and sample questions ChemC125 IUPUI Vinegar Questionnaire Sample Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. A few drops will indicate the endpoint of the titration (color. Calculate the volume of vinegar. To determine the percent by mass of acetic acid in vinegar. A titration setup is used for the. For precise measurement of the vinegar sample. In this. Vinegar Questionnaire Sample.
From www.studocu.com
Evaluation of vinegar sample quiz purpose the purpose of this Vinegar Questionnaire Sample For precise measurement of the vinegar sample. (2) to use the standardized sodium hydroxide solution. A few drops will indicate the endpoint of the titration (color. A titration setup is used for the. Calculate the volume of vinegar. A titration involves performing a controlled reaction between a solution of. (1) to standardize a solution of sodium hydroxide; To determine the. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample 1 Vinegar Vinegar Questionnaire Sample A titration setup is used for the. For precise measurement of the vinegar sample. To determine the percent by mass of acetic acid in vinegar. A few drops will indicate the endpoint of the titration (color. (2) to use the standardized sodium hydroxide solution. Calculate the volume of vinegar. The purpose of the vinegar analysis lab is to determine the. Vinegar Questionnaire Sample.
From www.chegg.com
Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 Vinegar Questionnaire Sample A titration involves performing a controlled reaction between a solution of. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. (2) to use the standardized sodium hydroxide solution. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample 1 Vinegar Vinegar Questionnaire Sample For precise measurement of the vinegar sample. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Calculate the volume of vinegar. (2) to use the standardized sodium hydroxide solution. A titration involves performing a controlled reaction between a solution of. Weigh each flask with the same balance and. Vinegar Questionnaire Sample.
From www.chegg.com
A. Preparation of Vinegar Sample Calculate the Vinegar Questionnaire Sample In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. (1) to standardize a solution of sodium hydroxide; For precise measurement of the vinegar sample. Calculate the volume of vinegar. (2) to use the standardized sodium hydroxide solution. Samples of one or two vinegars are analyzed for the amount. Vinegar Questionnaire Sample.
From www.numerade.com
SOLVED Table 2 Sample Sample 2 Average Snu Sml initial volume of Vinegar Questionnaire Sample Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. (1) to standardize a solution of sodium hydroxide; For precise measurement of the vinegar sample. A few drops will indicate the endpoint of the titration (color. A titration setup is used for the. In this experiment, a technique known as a titration will. Vinegar Questionnaire Sample.
From old.sermitsiaq.ag
Printable Questionnaire Maker Vinegar Questionnaire Sample A titration setup is used for the. (1) to standardize a solution of sodium hydroxide; (2) to use the standardized sodium hydroxide solution. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. Calculate the volume of vinegar. In this experiment, a technique known as a titration will be used to determine the. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 pH Vinegar Questionnaire Sample A titration involves performing a controlled reaction between a solution of. A titration setup is used for the. (2) to use the standardized sodium hydroxide solution. Calculate the volume of vinegar. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. A few drops will indicate the endpoint of. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Mass of Acetic Acid in Vinegar Sample (g)* Calculated Vinegar Questionnaire Sample Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. To determine the percent by mass of acetic acid in vinegar. Weigh each flask with the same balance and record the masses in your notebook. A titration involves performing a controlled reaction between a solution of. Calculate the volume of vinegar. A titration. Vinegar Questionnaire Sample.
From www.thinkswap.com
1.1 Vinegar analysis report/write up Biology Level 1 NCEA Thinkswap Vinegar Questionnaire Sample (2) to use the standardized sodium hydroxide solution. A titration setup is used for the. To determine the percent by mass of acetic acid in vinegar. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____. Vinegar Questionnaire Sample.
From mungfali.com
Sample Survey Questionnaire Format Vinegar Questionnaire Sample In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. A titration setup is used for the. (2) to use the standardized sodium hydroxide solution. A titration involves performing a controlled reaction. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample 1 Vinegar Vinegar Questionnaire Sample Weigh each flask with the same balance and record the masses in your notebook. Calculate the volume of vinegar. A titration involves performing a controlled reaction between a solution of. (2) to use the standardized sodium hydroxide solution. For precise measurement of the vinegar sample. To determine the percent by mass of acetic acid in vinegar. A few drops will. Vinegar Questionnaire Sample.
From slideplayer.com
Acids and Bases Titration of Vinegar ppt download Vinegar Questionnaire Sample Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. (2) to use the standardized sodium hydroxide solution. To determine the percent by mass of acetic acid in vinegar. A few drops will indicate the endpoint of the titration (color. In this experiment, a technique known as a titration will be used to. Vinegar Questionnaire Sample.
From www.slideshare.net
BILIMBI FRUIT VINEGAR A RESEARCH QUESTIONNAIRE PPT Vinegar Questionnaire Sample A titration setup is used for the. Weigh each flask with the same balance and record the masses in your notebook. A titration involves performing a controlled reaction between a solution of. For precise measurement of the vinegar sample. (1) to standardize a solution of sodium hydroxide; Calculate the volume of vinegar. To determine the percent by mass of acetic. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample 1 Vinegar Vinegar Questionnaire Sample Calculate the volume of vinegar. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. Weigh each flask with the same balance and record the masses in your notebook. (1) to standardize a solution of sodium hydroxide; For precise measurement of the vinegar sample. Samples of one or two. Vinegar Questionnaire Sample.
From www.studocu.com
Vinegar Analysis Sample Many common substances contain acids and Vinegar Questionnaire Sample Weigh each flask with the same balance and record the masses in your notebook. To determine the percent by mass of acetic acid in vinegar. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. A few drops will indicate the endpoint of the titration (color. The purpose of. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Data Table 3 Name of Sample Vinegar Vinegar Sample 1 Vinegar Questionnaire Sample Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. Weigh each flask with the same balance and record the masses in your notebook. A titration involves performing a controlled reaction between a solution of. A few drops will indicate the endpoint of the titration (color. (1) to standardize a solution of sodium. Vinegar Questionnaire Sample.
From www.researchgate.net
Sensory evaluation questionnaire for soup and vinegar. Download Vinegar Questionnaire Sample Calculate the volume of vinegar. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. A titration involves performing a controlled reaction between a solution of. (1) to standardize a solution of sodium hydroxide; (2) to use the standardized sodium hydroxide solution. A few drops will indicate the endpoint. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Data Table 2 Apple Cider Vinegar Vinegar Sample 1 Vinegar Questionnaire Sample For precise measurement of the vinegar sample. (1) to standardize a solution of sodium hydroxide; Calculate the volume of vinegar. A titration setup is used for the. A titration involves performing a controlled reaction between a solution of. Weigh each flask with the same balance and record the masses in your notebook. To determine the percent by mass of acetic. Vinegar Questionnaire Sample.
From www.numerade.com
SOLVED Vincaor Sample Vinegar Sample 2 Vinegar Sample 3 Nome of Vinegar Questionnaire Sample To determine the percent by mass of acetic acid in vinegar. A titration involves performing a controlled reaction between a solution of. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample.. Vinegar Questionnaire Sample.
From cengobjz.blob.core.windows.net
Psychology Questionnaire Examples at Joi Knight blog Vinegar Questionnaire Sample For precise measurement of the vinegar sample. (2) to use the standardized sodium hydroxide solution. A few drops will indicate the endpoint of the titration (color. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Calculate the volume of vinegar. A titration involves performing a controlled reaction between. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 Vinegar Questionnaire Sample A titration setup is used for the. A titration involves performing a controlled reaction between a solution of. (1) to standardize a solution of sodium hydroxide; Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 2 Vinegar Sample 3 4.878 5.028 4.85 Vinegar Questionnaire Sample A few drops will indicate the endpoint of the titration (color. For precise measurement of the vinegar sample. A titration setup is used for the. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. (2) to use the standardized sodium hydroxide solution. To determine the percent by mass of acetic acid in. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 Vinegar Questionnaire Sample A titration setup is used for the. A titration involves performing a controlled reaction between a solution of. The purpose of the vinegar analysis lab is to determine the percent by mass of acetic acid (ch 3 cooh¿in vinegar. (2) to use the standardized sodium hydroxide solution. In this experiment, a technique known as a titration will be used to. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample from Lab Kit Vinegar Questionnaire Sample (2) to use the standardized sodium hydroxide solution. To determine the percent by mass of acetic acid in vinegar. Samples of one or two vinegars are analyzed for the amount of _____ acid in the sample. A titration setup is used for the. For precise measurement of the vinegar sample. (1) to standardize a solution of sodium hydroxide; Calculate the. Vinegar Questionnaire Sample.
From www.chegg.com
Solved Name of Vinegar Sample Vinegar Sample 1 Vinegar Vinegar Questionnaire Sample A titration involves performing a controlled reaction between a solution of. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. (2) to use the standardized sodium hydroxide solution. Calculate the volume of vinegar. To determine the percent by mass of acetic acid in vinegar. Samples of one or. Vinegar Questionnaire Sample.