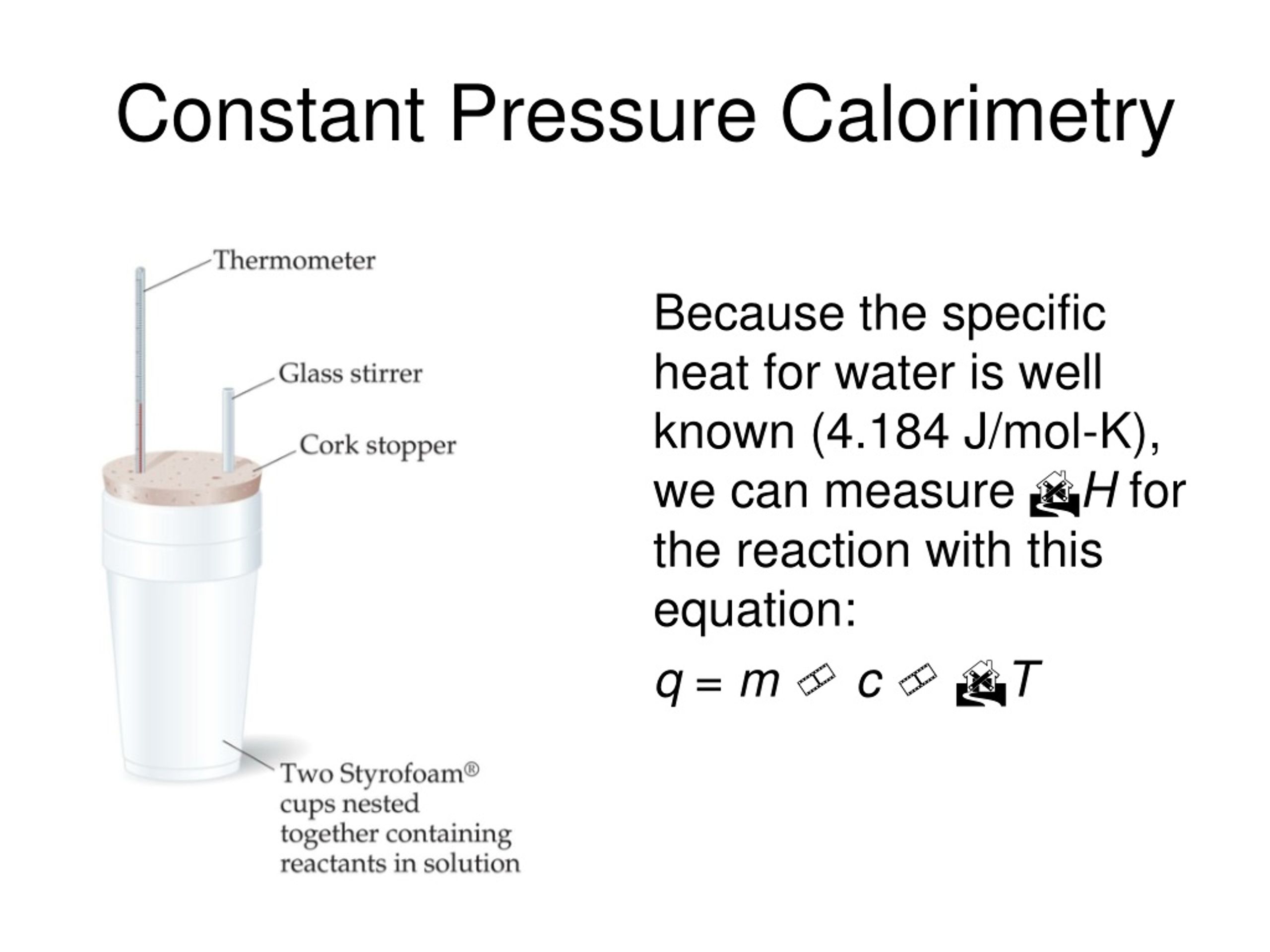
Calorimeter Constant And Temperature . Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. How to calculate a calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. The calibration gives you a number called the calorimeter constant. To do so, the heat is exchanged with a calibrated object (calorimeter). When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.slideserve.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object (calorimeter). The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree.
PPT Calorimetry PowerPoint Presentation, free download ID373573
Calorimeter Constant And Temperature When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The calibration gives you a number called the calorimeter constant. How to calculate a calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant And Temperature The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. For example, when an exothermic. For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimeter Constant And Temperature.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Calorimeter Constant And Temperature Apply the first law of thermodynamics to calorimetry. The calibration gives you a number called the calorimeter constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Constant And Temperature.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Constant And Temperature How to calculate a calorimeter constant. For example, when an exothermic. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. The calibration gives you a number called the calorimeter constant. Calorimetry is. Calorimeter Constant And Temperature.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Constant And Temperature It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. How to calculate a calorimeter constant. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. A calorimeter is a device used to measure. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. When 40.0 ml of water at 60.0 °c is added to 40.0. Calorimeter Constant And Temperature.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. It's the amount of heat. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant And Temperature When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. A calorimeter is a device used. Calorimeter Constant And Temperature.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. For example, when an exothermic. Apply the first law of thermodynamics to. Calorimeter Constant And Temperature.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. For example, when an exothermic. For example, when an exothermic. Calorimetry is used. Calorimeter Constant And Temperature.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby Calorimeter Constant And Temperature Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calibration gives you a number called the calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure. Calorimeter Constant And Temperature.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant And Temperature How to calculate a calorimeter constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calibration gives you a number called the. Calorimeter Constant And Temperature.
From saylordotorg.github.io
Calorimetry Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. How to calculate a calorimeter constant. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To. Calorimeter Constant And Temperature.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How to calculate a calorimeter constant.. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Calorimeter Constant And Temperature Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged. Calorimeter Constant And Temperature.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. For example, when an exothermic. To do so, the. Calorimeter Constant And Temperature.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Calorimeter Constant And Temperature The calibration gives you a number called the calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Constant And Temperature Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. For example, when. Calorimeter Constant And Temperature.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. The calibration gives you a number called the calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It's the amount of heat energy required to raise the temperature of the calorimeter by 1. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimeter Constant And Temperature For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calibration gives you a number called the calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c. Calorimeter Constant And Temperature.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Constant And Temperature The calibration gives you a number called the calorimeter constant. How to calculate a calorimeter constant. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It's. Calorimeter Constant And Temperature.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Calorimeter Constant And Temperature How to calculate a calorimeter constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. When. Calorimeter Constant And Temperature.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimeter Constant And Temperature To do so, the heat is exchanged with a calibrated object (calorimeter). The calibration gives you a number called the calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. How to calculate a calorimeter constant. A calorimeter is a device used to measure the amount of heat. Calorimeter Constant And Temperature.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimeter Constant And Temperature How to calculate a calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so,. Calorimeter Constant And Temperature.
From 2012books.lardbucket.org
Calorimetry Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). When 40.0 ml of water at 60.0 °c is. Calorimeter Constant And Temperature.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calibration gives you a number called the calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1. Calorimeter Constant And Temperature.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Constant And Temperature To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. When 40.0. Calorimeter Constant And Temperature.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Constant And Temperature When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold. Calorimeter Constant And Temperature.
From www.chegg.com
Solved 2. A calorimeter can be calibrated by applying heat Calorimeter Constant And Temperature Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred. Calorimeter Constant And Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant And Temperature For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The. Calorimeter Constant And Temperature.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. How to calculate a calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic. When 40.0 ml of. Calorimeter Constant And Temperature.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL Calorimeter Constant And Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It's the. Calorimeter Constant And Temperature.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Constant And Temperature Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. The calibration gives you a number called the calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. How to calculate a calorimeter constant. For example, when an. Calorimeter Constant And Temperature.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Constant And Temperature How to calculate a calorimeter constant. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. For example, when an exothermic.. Calorimeter Constant And Temperature.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Constant And Temperature Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calibration gives you a number called the calorimeter constant. For example, when. Calorimeter Constant And Temperature.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Constant And Temperature A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. When 40.0 ml of water at. Calorimeter Constant And Temperature.