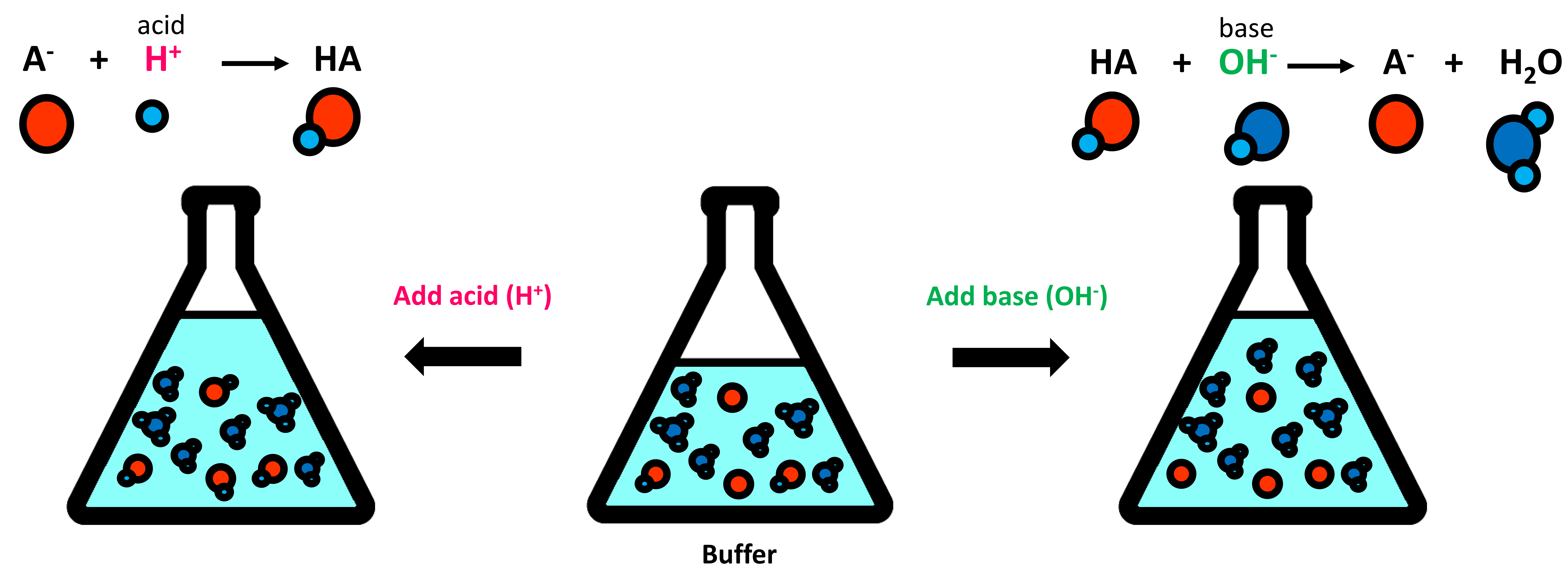
Properties Of Buffer Solutions Lab . A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. It is able to neutralize small amounts of added acid or base, thus. Buffers are solutions that resist a change in ph after adding an acid or a base. An effective buffer system contains. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Adding a strong electrolyte that. Equation 5 is sometimes known as the buffer equation; Buffer solutions are known to be resistant to ph changes. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer solutions contain a weak acid and its conjugate base, or a weak. Buffers resist changes in ph when acids or bases are added to them. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. A buffer solution can resist ph change because of an equilibrium between the acid. It provides the key to calculating the properties of buffer solutions.
from design.udlvirtual.edu.pe
Equation 5 is sometimes known as the buffer equation; A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. An effective buffer system contains. Buffers resist changes in ph when acids or bases are added to them. Buffer solutions contain a weak acid and its conjugate base, or a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Buffers are solutions that resist a change in ph after adding an acid or a base.
How Does A Circular Buffer Work Design Talk
Properties Of Buffer Solutions Lab Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize small amounts of added acid or base, thus. Buffer solutions contain a weak acid and its conjugate base, or a weak. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. An effective buffer system contains. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers resist changes in ph when acids or bases are added to them. Adding a strong electrolyte that. Equation 5 is sometimes known as the buffer equation; A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. A buffer solution can resist ph change because of an equilibrium between the acid. Buffer solutions are known to be resistant to ph changes. Examination of buffer solutions & A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It provides the key to calculating the properties of buffer solutions. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)).
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Properties Of Buffer Solutions Lab A buffer solution can resist ph change because of an equilibrium between the acid. It provides the key to calculating the properties of buffer solutions. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. Buffers resist changes in ph when acids or bases are added to them. Buffer solutions contain a. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved Please fill in the data table and answer all of the Properties Of Buffer Solutions Lab A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. An effective buffer system contains. Buffers resist changes in ph when acids or bases are added to them. Adding a strong electrolyte that. Equation. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved PROPERTIES AND PREPARATION OF BUFFERS INLAB Properties Of Buffer Solutions Lab Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer solution can resist ph change because of an equilibrium between the acid. Adding a strong electrolyte that. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). An effective buffer system contains. A buffer solution consists of a conjugate acid/base. Properties Of Buffer Solutions Lab.
From www.youtube.com
Preparation and Properties of Buffer Solution Chemical Equilibrium Properties Of Buffer Solutions Lab Adding a strong electrolyte that. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffer solutions are known to be resistant to ph changes. Equation 5 is sometimes known as the buffer equation; Buffers. Properties Of Buffer Solutions Lab.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Properties Of Buffer Solutions Lab A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Examination of buffer solutions & Adding a strong electrolyte that. It provides the key to calculating the properties of buffer solutions. A buffer solution can resist ph change because of an equilibrium between the acid. Equation 5 is sometimes known. Properties Of Buffer Solutions Lab.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool Properties Of Buffer Solutions Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. Equation 5 is sometimes known as the buffer equation; A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Buffers resist changes in ph when acids or bases. Properties Of Buffer Solutions Lab.
From www.flinnsci.ca
FlinnPREP™ Inquiry Labs for AP® Chemistry Properties of Buffer Properties Of Buffer Solutions Lab A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. Equation 5 is sometimes known as the buffer equation; Adding a strong electrolyte that. Examination of buffer solutions & It provides the key to calculating the properties of buffer solutions. Buffers are solutions that resist a change in ph after adding an. Properties Of Buffer Solutions Lab.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Properties Of Buffer Solutions Lab Buffers resist changes in ph when acids or bases are added to them. It provides the key to calculating the properties of buffer solutions. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. A buffer solution consists of a conjugate acid/base pair, so it maintains. Properties Of Buffer Solutions Lab.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Properties Of Buffer Solutions Lab Equation 5 is sometimes known as the buffer equation; Examination of buffer solutions & It provides the key to calculating the properties of buffer solutions. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. It is able to neutralize small amounts of added acid or base, thus. A buffer solution can. Properties Of Buffer Solutions Lab.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Properties Of Buffer Solutions Lab Equation 5 is sometimes known as the buffer equation; A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution’s ph, on the other hand, can vary depending on. Properties Of Buffer Solutions Lab.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Properties Of Buffer Solutions Lab Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Examination of buffer solutions &. Properties Of Buffer Solutions Lab.
From sciencing.com
How to Make a Citric Acid Buffer Solution Sciencing Properties Of Buffer Solutions Lab Examination of buffer solutions & An effective buffer system contains. It is able to neutralize small amounts of added acid or base, thus. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. Buffer solutions contain. Properties Of Buffer Solutions Lab.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Properties Of Buffer Solutions Lab Buffer solutions are known to be resistant to ph changes. A buffer solution can resist ph change because of an equilibrium between the acid. Buffer solutions contain a weak acid and its conjugate base, or a weak. Adding a strong electrolyte that. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon.. Properties Of Buffer Solutions Lab.
From www.youtube.com
LAB PROPERTIES OF BUFFER SOLUTIONS YouTube Properties Of Buffer Solutions Lab Equation 5 is sometimes known as the buffer equation; Buffer solutions contain a weak acid and its conjugate base, or a weak. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers resist changes in ph when acids or bases are added to them. Adding a strong electrolyte that.. Properties Of Buffer Solutions Lab.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Properties Of Buffer Solutions Lab Adding a strong electrolyte that. It is able to neutralize small amounts of added acid or base, thus. An effective buffer system contains. Examination of buffer solutions & A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Buffers are solutions that resist a change in ph after adding an acid or. Properties Of Buffer Solutions Lab.
From www.studocu.com
Properties of Buffer Solutions 2 Results General Chemistry Two SCC Properties Of Buffer Solutions Lab A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Equation 5 is sometimes known as the buffer equation; A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions. Properties Of Buffer Solutions Lab.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Properties Of Buffer Solutions Lab It is able to neutralize small amounts of added acid or base, thus. Examination of buffer solutions & A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Buffer solutions are known to. Properties Of Buffer Solutions Lab.
From studylib.net
pH Properties of Buffer Solutions Properties Of Buffer Solutions Lab Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Equation 5 is sometimes known as the buffer equation; Buffer solutions are known to be resistant to ph changes. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer solution consists of a weak acid and its conjugate base or a weak. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Properties Of Buffer Solutions Lab Buffers resist changes in ph when acids or bases are added to them. Examination of buffer solutions & A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. An effective buffer system contains. Equation 5 is sometimes known as the buffer equation; A buffer solution consists of a conjugate acid/base. Properties Of Buffer Solutions Lab.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Properties Of Buffer Solutions Lab A buffer solution can resist ph change because of an equilibrium between the acid. Buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or. Properties Of Buffer Solutions Lab.
From www.slideshare.net
Properties of buffers Properties Of Buffer Solutions Lab Buffer solutions are known to be resistant to ph changes. Adding a strong electrolyte that. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. It provides the key to calculating the properties of buffer solutions. Buffers resist. Properties Of Buffer Solutions Lab.
From studylib.net
Preparation and Properties of Buffer Solutions Properties Of Buffer Solutions Lab Adding a strong electrolyte that. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Examination of buffer solutions & Buffers are solutions that resist a change in ph after adding an acid or a base. Buffer solutions are known to be resistant to ph changes. A buffer solution can resist. Properties Of Buffer Solutions Lab.
From www.slideserve.com
PPT AP Chemistry PowerPoint Presentation, free download ID3198692 Properties Of Buffer Solutions Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It provides the key to calculating the properties of buffer solutions. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. Adding a strong electrolyte that. It is able to neutralize small amounts. Properties Of Buffer Solutions Lab.
From studylib.net
Properties of Buffer Solutions Intro Properties Of Buffer Solutions Lab Examination of buffer solutions & Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. A buffer solution. Properties Of Buffer Solutions Lab.
From www.studocu.com
Studying the properties of Buffer Solution Material 0 Acetic Acid Properties Of Buffer Solutions Lab Buffers resist changes in ph when acids or bases are added to them. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. A buffer solution can resist ph change because of an equilibrium between the acid. Buffers are solutions that resist a change in ph after adding an acid or a. Properties Of Buffer Solutions Lab.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Properties Of Buffer Solutions Lab An effective buffer system contains. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon. Buffers resist changes in ph when acids or bases are added to them. Buffer solutions are known to be resistant to ph changes. A buffer is a solution that can resist ph change upon the addition of. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved PART D. Properties of Buffer Solution Solution Buffer Properties Of Buffer Solutions Lab A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. A buffer solution can resist ph change because of an equilibrium between the acid. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffer solutions are known to be resistant to ph changes. Buffers contain. Properties Of Buffer Solutions Lab.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Properties Of Buffer Solutions Lab Buffer solutions are known to be resistant to ph changes. Adding a strong electrolyte that. Buffer solutions contain a weak acid and its conjugate base, or a weak. It provides the key to calculating the properties of buffer solutions. It is able to neutralize small amounts of added acid or base, thus. A buffer solution consists of a weak acid. Properties Of Buffer Solutions Lab.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Properties Of Buffer Solutions Lab Adding a strong electrolyte that. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution can resist ph change because of an equilibrium between the acid. Buffer solutions are known to be resistant to ph changes. It provides the key to calculating the properties of buffer solutions.. Properties Of Buffer Solutions Lab.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Properties Of Buffer Solutions Lab Buffer solutions are known to be resistant to ph changes. Buffers resist changes in ph when acids or bases are added to them. Adding a strong electrolyte that. It provides the key to calculating the properties of buffer solutions. It is able to neutralize small amounts of added acid or base, thus. A buffer solution consists of a conjugate acid/base. Properties Of Buffer Solutions Lab.
From www.youtube.com
Lab 8 Acids, Bases, and Buffers experiment YouTube Properties Of Buffer Solutions Lab A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Examination of buffer solutions & It is able to neutralize small amounts of added acid or base, thus. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It provides the key to calculating the properties of buffer. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved Observations and Calculations pH Buffers and Their Properties Of Buffer Solutions Lab It provides the key to calculating the properties of buffer solutions. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. It is able to neutralize small amounts of added acid or base, thus. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer. Properties Of Buffer Solutions Lab.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Properties Of Buffer Solutions Lab A buffer solution can resist ph change because of an equilibrium between the acid. It provides the key to calculating the properties of buffer solutions. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. Buffers resist changes in ph when acids or bases are added. Properties Of Buffer Solutions Lab.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Properties Of Buffer Solutions Lab Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer solution’s ph, on the other hand, can vary depending on how much strong acid or strong. An effective buffer system contains. Examination of buffer solutions & Buffer solutions contain a weak acid and its conjugate base, or a weak. Buffers contain a. Properties Of Buffer Solutions Lab.
From www.chegg.com
Solved Exp 25. pH MeasurementsBuffers and Their Properties. Properties Of Buffer Solutions Lab Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Equation 5 is sometimes known as the buffer equation; An effective buffer system contains. A buffer solution consists of a weak acid and its conjugate base or a weak base and its. Properties Of Buffer Solutions Lab.