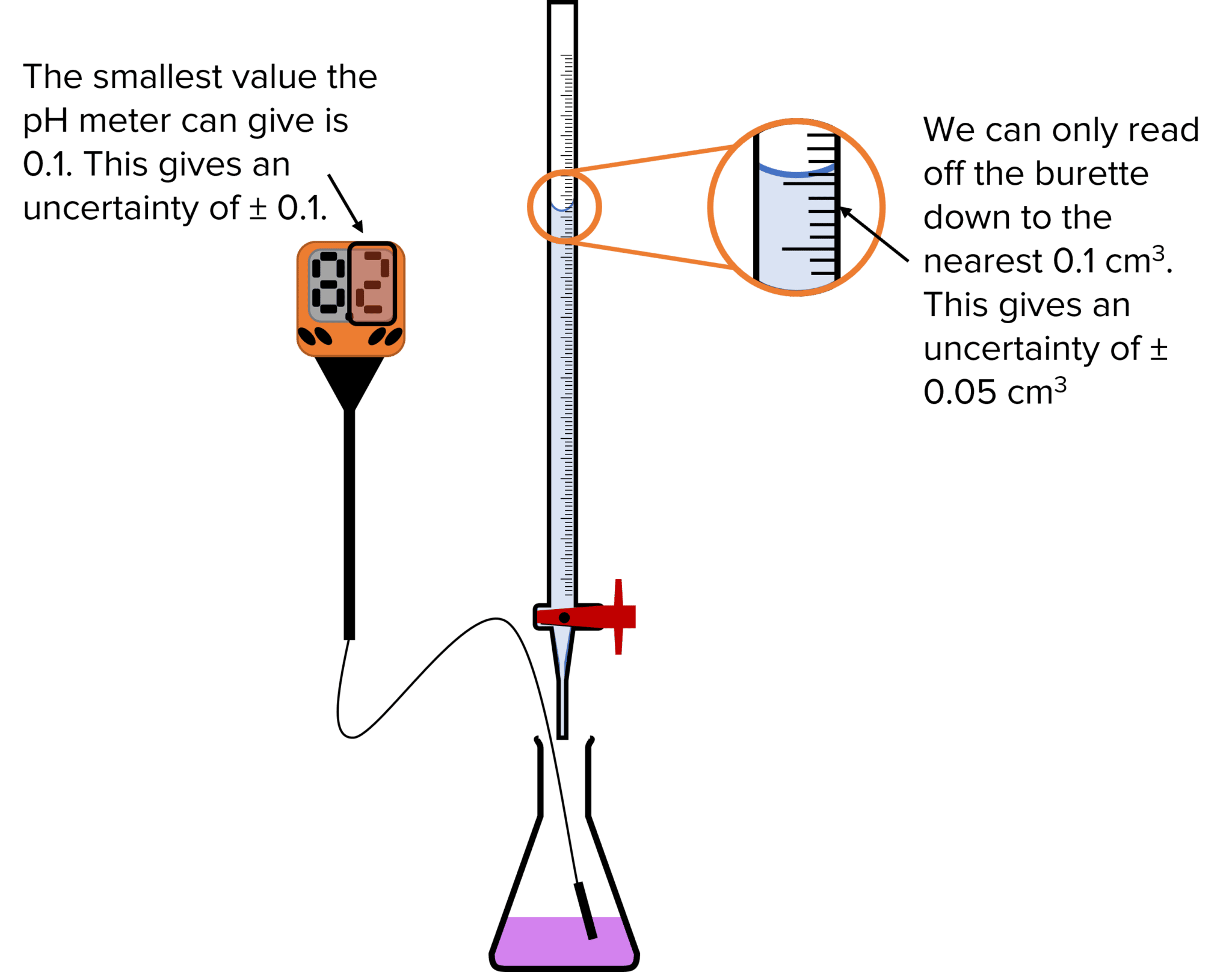
Titration Equation Problems . This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The example below demonstrates the. Titrations are a method of analysing the concentration of solutions.
from giogcqhar.blob.core.windows.net
This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titrations are a method of analysing the concentration of solutions. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The example below demonstrates the.
Titration Definition Psychology at ster blog
Titration Equation Problems The example below demonstrates the. Titrations are a method of analysing the concentration of solutions. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Equation Problems Titrations are a method of analysing the concentration of solutions. The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Titration Equation Problems.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Equation Problems This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titrations are a method of analysing the concentration of solutions. The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of. Titration Equation Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Equation Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we will see how to perform calculations to predict the ph at any. Titration Equation Problems.
From docworksheet.com
Gcse Titration Calculations Worksheet Titration Equation Problems The example below demonstrates the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titrations are a method of analysing the concentration of solutions. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Equation Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Equation Problems The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Titration Equation Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Equation Problems This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The example below demonstrates the. The above equation. Titration Equation Problems.
From mungfali.com
Acid Base Titration Calculation Titration Equation Problems The example below demonstrates the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This webpage describes. Titration Equation Problems.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via Titration Equation Problems Titrations are a method of analysing the concentration of solutions. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. The example below demonstrates the. A. Titration Equation Problems.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Equation Problems The example below demonstrates the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In this section,. Titration Equation Problems.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. The above equation works only for neutralizations in. Titration Equation Problems.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Equation Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titrations are a method of analysing the concentration of solutions. A titration is a volumetric technique. Titration Equation Problems.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Equation Problems A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titrations are a method. Titration Equation Problems.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.. Titration Equation Problems.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Titration Equation Problems The example below demonstrates the. Titrations are a method of analysing the concentration of solutions. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Equation Problems.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titrations are a method of analysing the concentration. Titration Equation Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Equation Problems Titrations are a method of analysing the concentration of solutions. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and. Titration Equation Problems.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Equation Problems This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The example below demonstrates the. A titration is. Titration Equation Problems.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. The example below demonstrates the. Titrations are a. Titration Equation Problems.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. The above equation works only for neutralizations in. Titration Equation Problems.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in. Titration Equation Problems.
From giogcqhar.blob.core.windows.net
Titration Definition Psychology at ster blog Titration Equation Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The example below demonstrates the. In this section,. Titration Equation Problems.
From www.numerade.com
Challenge Problem. Sulfide ion ( S^2 ) is formed in wastewater by the Titration Equation Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This webpage describes a procedure called titration, which. Titration Equation Problems.
From tukioka-clinic.com
👍 Solve titration problems. How to solve titration problems step by Titration Equation Problems Titrations are a method of analysing the concentration of solutions. The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Equation Problems.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Equation Problems A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we. Titration Equation Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Equation Problems A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we. Titration Equation Problems.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Equation Problems Titrations are a method of analysing the concentration of solutions. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This webpage describes a procedure called titration, which can be used to find the molarity of a solution. Titration Equation Problems.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Equation Problems Titrations are a method of analysing the concentration of solutions. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or. Titration Equation Problems.
From hxeypsqim.blob.core.windows.net
Titration Problems Explained at Della Gibbs blog Titration Equation Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titrations are a method of analysing the concentration of solutions. The example below demonstrates the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Equation Problems.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Equation Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration is a volumetric technique in which. Titration Equation Problems.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Equation Problems A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the.. Titration Equation Problems.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration Equation Problems The example below demonstrates the. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Titrations are a. Titration Equation Problems.