Acetic Acid + Water . When acetic acid is dissolved in water there is an equilibrium reaction: Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. What is the concentration of commercial. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. When we add acetic acid to water, it ionizes to a small extent according to the equation: It has a pungent smell. Vinegar is primarily an aqueous solution of acetic acid.
from www.transtutors.com
Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When acetic acid is dissolved in water there is an equilibrium reaction: When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. When we add acetic acid to water, it ionizes to a small extent according to the equation: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar is primarily an aqueous solution of acetic acid. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; It has a pungent smell. What is the concentration of commercial.
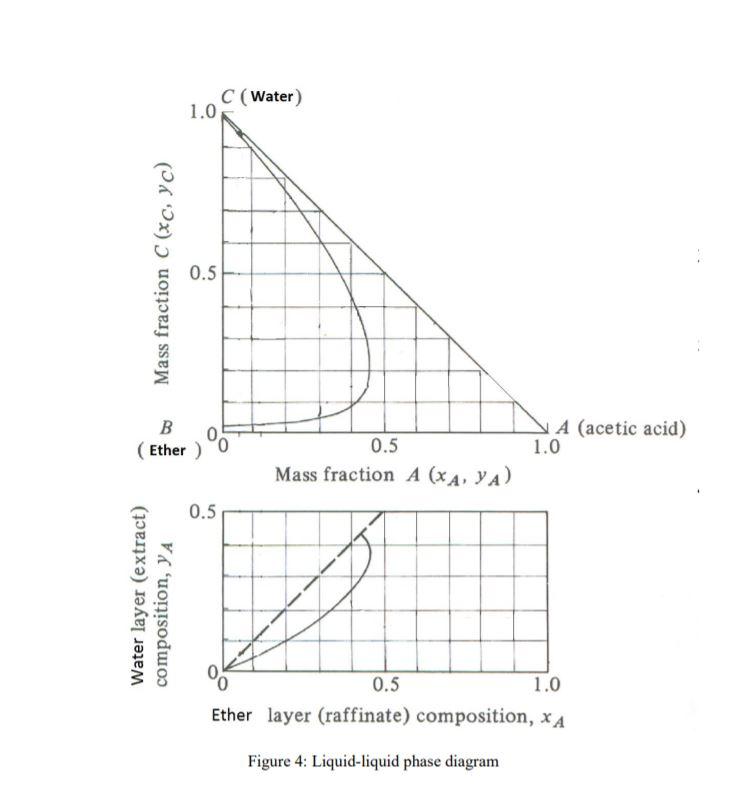
(Solved) (a) Acetic acid is extracted from isopropyl ether using pure
Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When we add acetic acid to water, it ionizes to a small extent according to the equation: Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. It has a pungent smell. Vinegar is primarily an aqueous solution of acetic acid. When acetic acid is dissolved in water there is an equilibrium reaction: What is the concentration of commercial. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Melting point 16.6 °c [61.9 °f]) that is completely miscible with.
From pdfprof.com
acetic anhydride is obtained by the reaction of acetic acid and Acetic Acid + Water When acetic acid is dissolved in water there is an equilibrium reaction: Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. It has a pungent smell. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar is primarily an aqueous solution of acetic acid. Pure acetic. Acetic Acid + Water.
From www.showme.com
Net ionic equations acetic acid and strontium hydroxide Science Acetic Acid + Water When acetic acid is dissolved in water there is an equilibrium reaction: Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a. Acetic Acid + Water.
From www.tessshebaylo.com
Write The Balanced Net Ionic Equation For Dissociation Of Acetic Acid Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; Vinegar is primarily an aqueous solution of acetic acid. Melting point 16.6 °c [61.9 °f]) that. Acetic Acid + Water.
From www.chegg.com
Solved Water is used to extract acetic acid from a mixture Acetic Acid + Water When we add acetic acid to water, it ionizes to a small extent according to the equation: Vinegar is primarily an aqueous solution of acetic acid. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; It has a pungent smell. When acetic acid, ch3cooh, which is a weak acid, is. Acetic Acid + Water.
From www.coursehero.com
Acetic acid Water Isopropyl ether system LLE Data for Acetic Acetic Acid + Water Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. When we add acetic acid to water, it ionizes to a small extent according to the equation: When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0. Acetic Acid + Water.
From pubs.usgs.gov
USGS OFR01041 Procedures Acetic Acid Treatment to Remove Carbonates Acetic Acid + Water Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Vinegar is. Acetic Acid + Water.
From www.myxxgirl.com
Acetic Acid Phase Diagram My XXX Hot Girl Acetic Acid + Water Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar is primarily an aqueous solution of acetic acid. What is the concentration of commercial. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When acetic acid is dissolved in water there is an equilibrium reaction: When we add. Acetic Acid + Water.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid + Water Vinegar is primarily an aqueous solution of acetic acid. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. What is the concentration of commercial. When we add acetic acid to water, it ionizes to a small extent according to the equation: Commercial vinegar typically. Acetic Acid + Water.
From www.researchgate.net
Phase diagram for acetic acid and water. 5 Download Scientific Diagram Acetic Acid + Water Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When we add acetic acid to water, it ionizes to a small extent according to the equation: What is the concentration of commercial. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar is primarily an aqueous solution of acetic acid. When acetic acid,. Acetic Acid + Water.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a small extent according to the. Acetic Acid + Water.
From sciencenotes.org
What Is Glacial Acetic Acid? Acetic Acid + Water Vinegar is primarily an aqueous solution of acetic acid. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. When acetic acid is dissolved in water there is an equilibrium reaction: Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Melting point 16.6 °c [61.9 °f]) that. Acetic Acid + Water.
From www.wikidoc.org
Acetic acid wikidoc Acetic Acid + Water Melting point 16.6 °c [61.9 °f]) that is completely miscible with. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. It has a pungent smell. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f];. Acetic Acid + Water.
From agenciademodelospontealta.blogspot.com
21 Luxury Acetic Acid Process Flow Diagram Acetic Acid + Water Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a small extent according to the equation: Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of. Acetic Acid + Water.
From www.youtube.com
Water , Acetic Acid and Chloroform system 01 PHASE RULE19 YouTube Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. It has a pungent smell. When we add acetic acid to water, it ionizes to a small extent according to the equation: Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid. Acetic Acid + Water.
From www.alamy.com
Acetic acid (ethanoic acid) organic chemistry lesson Stock Vector Acetic Acid + Water What is the concentration of commercial. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; Vinegar is primarily an aqueous solution of acetic acid. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When we add acetic acid to water, it ionizes to a small. Acetic Acid + Water.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Acetic Acid + Water Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When we add acetic acid to water, it ionizes to a small extent according to the equation: Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; What is the concentration of commercial. Acetic acid (\(\ce{ch3co2h}\)) is. Acetic Acid + Water.
From www.answersarena.com
[Solved] Q4 Acetic acid and water react to form hydronium Acetic Acid + Water Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a small extent according to the equation: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar is primarily an aqueous solution of acetic acid. Commercial vinegar typically contains. Acetic Acid + Water.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Acetic Acid + Water Vinegar is primarily an aqueous solution of acetic acid. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; It has a pungent smell. What is the concentration of commercial. When acetic acid is dissolved in water there is an equilibrium reaction: Melting point 16.6 °c [61.9 °f]) that is completely. Acetic Acid + Water.
From learnphysics-dhruv.blogspot.com
Physics Learn Physical and chemical properties of acetic acid. Science Acetic Acid + Water Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the. Acetic Acid + Water.
From www.vectorstock.com
Formula and model of acetic acid molecule Vector Image Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Vinegar is primarily an aqueous solution of acetic acid. It has a pungent smell. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. What is the concentration of commercial. Commercial vinegar typically contains. Acetic Acid + Water.
From www.youtube.com
Ethanoic Acid + Water = ?? YouTube Acetic Acid + Water What is the concentration of commercial. When we add acetic acid to water, it ionizes to a small extent according to the equation: When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Pure acetic acid, often called. Acetic Acid + Water.
From ar.inspiredpencil.com
Acetic Anhydride And Water Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. When we add acetic acid to water, it ionizes to a small extent according to the equation: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. It has a pungent smell. Vinegar is. Acetic Acid + Water.
From myprojectideas.com
Properties of Acetic Acid Science Experiment My Project Ideas Acetic Acid + Water Vinegar is primarily an aqueous solution of acetic acid. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a small extent according to the equation: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. When acetic acid, ch3cooh,. Acetic Acid + Water.
From mavink.com
Acetic Acid Phase Diagram Acetic Acid + Water Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. What is the concentration of commercial. Vinegar is primarily an aqueous solution of acetic acid. It has a pungent smell. Pure. Acetic Acid + Water.
From www.thoughtco.com
10 Common Acids and Chemical Structures Acetic Acid + Water Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Vinegar is primarily an aqueous solution of acetic acid. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; What is the concentration of commercial. When acetic acid, ch3cooh, which. Acetic Acid + Water.
From betance86662.blogspot.com
Acetic Acid To Acetic Anhydride Betance86662 Acetic Acid + Water Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. What is the concentration of commercial. Vinegar is primarily an aqueous solution of acetic acid. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid. Acetic Acid + Water.
From www.youtube.com
How to Write the Formula for Acetic acid YouTube Acetic Acid + Water Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. It has a pungent smell. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. When we add acetic acid to water, it ionizes. Acetic Acid + Water.
From selfdirectedce.com
Is CH3COOH (Acetic acid) Soluble or Insoluble in Water? สรุปข้อมูลที่ Acetic Acid + Water Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Pure acetic acid,. Acetic Acid + Water.
From pdfprof.com
acidic hydrolysis of ethyl acetate Acetic Acid + Water It has a pungent smell. What is the concentration of commercial. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. When acetic acid is dissolved in water there is an equilibrium reaction: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Vinegar. Acetic Acid + Water.
From www.slideserve.com
PPT Melting Points and Mixed Melting Points PowerPoint Presentation Acetic Acid + Water It has a pungent smell. Vinegar is primarily an aqueous solution of acetic acid. Melting point 16.6 °c [61.9 °f]) that is completely miscible with. When we add acetic acid to water, it ionizes to a small extent according to the equation: When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come. Acetic Acid + Water.
From www.peptidegold.com
Acetic Acid Water 10ml Peptide Gold Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. What is the concentration of commercial. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. Pure acetic acid, often called glacial acetic acid,. Acetic Acid + Water.
From www.tessshebaylo.com
Ionization Of Acetic Acid Equilibrium Equation Tessshebaylo Acetic Acid + Water Melting point 16.6 °c [61.9 °f]) that is completely miscible with. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. What is the concentration of commercial. When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0. Acetic Acid + Water.
From www.researchgate.net
TLC carried out using ethyl acetatewateracetic acid (1.511) on the Acetic Acid + Water Vinegar is primarily an aqueous solution of acetic acid. What is the concentration of commercial. When we add acetic acid to water, it ionizes to a small extent according to the equation: When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. It has a. Acetic Acid + Water.
From pdfprof.com
acetic anhydride boiling point and density Acetic Acid + Water When acetic acid, ch3cooh, which is a weak acid, is mixed with water, some of the h's come off of the cooh group of the. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. It has a pungent smell. When we add acetic acid to water, it ionizes to a small extent according to the. Acetic Acid + Water.
From www.transtutors.com
(Solved) (a) Acetic acid is extracted from isopropyl ether using pure Acetic Acid + Water Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °c [244.2 °f]; When we add acetic acid to water, it ionizes to a small extent according to the equation: When acetic acid is dissolved in water there is an equilibrium reaction: Melting point 16.6 °c [61.9 °f]) that is completely miscible with. When. Acetic Acid + Water.