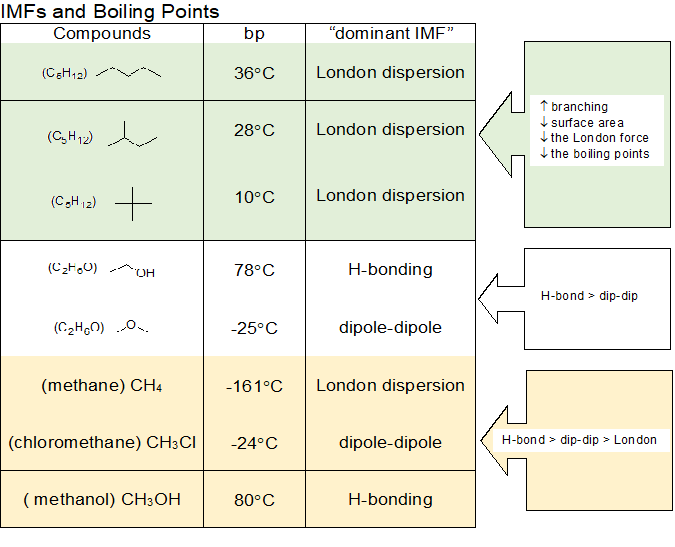
Boiling Point Trends Of Organic Compounds . Boiling points increase as the number of carbons is increased. Predict the relative boil points of organic compounds. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The intermolecular forces increase with increasing polarization (i.e. The stronger the imfs, the lower the vapor pressure of the. Boiling points are a measure of intermolecular forces. Physical properties of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points.
from estudarpara.com
An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Intermolecular forces (imfs) can be used to predict relative boiling points. Difference in electronegativity) of bonds. Physical properties of organic compounds. Boiling points are a measure of intermolecular forces. The size of a molecule influences its. The stronger the imfs, the lower the vapor pressure of the. The intermolecular forces increase with increasing polarization (i.e. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points increase as the number of carbons is increased.
Predict the trend in boiling points for the following compounds (1
Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the imfs, the lower the vapor pressure of the. The intermolecular forces increase with increasing polarization (i.e. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points increase as the number of carbons is increased. Intermolecular forces (imfs) can be used to predict relative boiling points. Difference in electronegativity) of bonds. Physical properties of organic compounds. The size of a molecule influences its. Boiling points are a measure of intermolecular forces. Predict the relative boil points of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many.
From www.chemistrysteps.com
Boiling Point and Melting Point Practice Problems Chemistry Steps Boiling Point Trends Of Organic Compounds Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization (i.e. Boiling points are a measure of intermolecular forces. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. Boiling points increase as the number of carbons is increased. Physical properties of organic compounds. An understanding of the various types. Boiling Point Trends Of Organic Compounds.
From www.youtube.com
Boiling Point of Organic Compounds YouTube Boiling Point Trends Of Organic Compounds Boiling points increase as the number of carbons is increased. Boiling points are a measure of intermolecular forces. The size of a molecule influences its. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The intermolecular forces increase with increasing polarization (i.e. Predict the relative boil points of organic compounds. An. Boiling Point Trends Of Organic Compounds.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID9293548 Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The intermolecular forces increase with increasing polarization (i.e. Difference in electronegativity) of bonds. The stronger the imfs, the lower the vapor pressure of the. The size of a molecule influences its.. Boiling Point Trends Of Organic Compounds.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:4.2.1 Crude Oil Boiling Point Trends Of Organic Compounds An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Predict the relative boil points of organic compounds. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. An understanding of the various types of noncovalent forces allows us to explain, on a molecular. Boiling Point Trends Of Organic Compounds.
From www.engineeringtoolbox.com
Melting points of hydrocarbons, alcohols and acids Boiling Point Trends Of Organic Compounds Physical properties of organic compounds. Predict the relative boil points of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Difference in electronegativity) of bonds. Intermolecular forces (imfs) can be used to predict relative boiling points. Boiling points increase as the number of carbons is increased. An understanding of. Boiling Point Trends Of Organic Compounds.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds Boiling points increase as the number of carbons is increased. The size of a molecule influences its. Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization (i.e. The stronger the imfs, the lower the vapor pressure of the. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Physical. Boiling Point Trends Of Organic Compounds.
From www.engineeringtoolbox.com
Boiling points of hydrocarbons, alcohols and acids Boiling Point Trends Of Organic Compounds Physical properties of organic compounds. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. Predict the relative boil points of organic compounds. Boiling points are a measure of intermolecular forces. The stronger the imfs, the lower the vapor pressure of the. An understanding of the various types of noncovalent forces allows. Boiling Point Trends Of Organic Compounds.
From mavink.com
Organic Chemistry Boiling Point Chart Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. Predict the relative boil points of organic compounds. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points are a measure of intermolecular forces. The size of a molecule influences its. An understanding of. Boiling Point Trends Of Organic Compounds.
From www.youtube.com
Explaining Boiling Point (Simple Covalent, Intermolecular Forces Boiling Point Trends Of Organic Compounds Boiling points are a measure of intermolecular forces. The intermolecular forces increase with increasing polarization (i.e. Physical properties of organic compounds. Boiling points increase as the number of carbons is increased. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Predict the relative boil points of. Boiling Point Trends Of Organic Compounds.
From wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Boiling Point Trends Of Organic Compounds Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization (i.e. Boiling points are a measure of intermolecular forces. Intermolecular forces (imfs) can be used to predict relative boiling points. The size of a molecule influences its. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The stronger the. Boiling Point Trends Of Organic Compounds.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds The size of a molecule influences its. Predict the relative boil points of organic compounds. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points increase as the number of carbons is increased. An understanding of the various types of noncovalent forces allows us to. Boiling Point Trends Of Organic Compounds.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID3665787 Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. Physical properties of organic compounds. Boiling points are a measure of intermolecular forces. Predict the relative boil points of organic compounds. Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization (i.e. The size of a molecule influences its. The stronger the imfs, the lower the vapor. Boiling Point Trends Of Organic Compounds.
From www.conquerhsc.com
HSC Chemistry Module 7 Inquiry Question 2 Boiling Point Trends Of Organic Compounds Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization (i.e. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Physical properties of organic compounds. Intermolecular forces (imfs) can be used to predict relative boiling points. Predict the relative boil points of organic compounds. The stronger the imfs, the. Boiling Point Trends Of Organic Compounds.
From nelsonewawalters.blogspot.com
Boiling Point of Alcohol NelsonewaWalters Boiling Point Trends Of Organic Compounds The intermolecular forces increase with increasing polarization (i.e. The size of a molecule influences its. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Difference in electronegativity) of bonds. Boiling points are a measure of intermolecular forces. The stronger the imfs, the lower the vapor pressure of the. Predict the relative. Boiling Point Trends Of Organic Compounds.
From desklib.com
Trends in Boiling Points of Organic Compounds Desklib Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. Difference in electronegativity) of bonds. The stronger the imfs, the lower the vapor pressure of the. The intermolecular forces increase with increasing polarization (i.e. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Physical properties of organic compounds. Boiling points. Boiling Point Trends Of Organic Compounds.
From www.nagwa.com
Question Video Identifying the Organic Molecule with the Highest Boiling Point Trends Of Organic Compounds Difference in electronegativity) of bonds. Intermolecular forces (imfs) can be used to predict relative boiling points. The intermolecular forces increase with increasing polarization (i.e. Boiling points are a measure of intermolecular forces. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. An understanding of the various types of noncovalent forces allows. Boiling Point Trends Of Organic Compounds.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds Intermolecular forces (imfs) can be used to predict relative boiling points. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points increase as the number of carbons is increased. The stronger the imfs, the lower the vapor pressure of the. An understanding of the various types of noncovalent forces allows. Boiling Point Trends Of Organic Compounds.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID2942238 Boiling Point Trends Of Organic Compounds Physical properties of organic compounds. Difference in electronegativity) of bonds. Intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the imfs, the lower the vapor pressure of the. Boiling points are a measure of intermolecular forces. The intermolecular forces increase with increasing polarization (i.e. The size of a molecule influences its. Boiling points increase as the. Boiling Point Trends Of Organic Compounds.
From wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Boiling Point Trends Of Organic Compounds Boiling points increase as the number of carbons is increased. Physical properties of organic compounds. The intermolecular forces increase with increasing polarization (i.e. Predict the relative boil points of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Difference in electronegativity) of bonds. The size of a molecule influences. Boiling Point Trends Of Organic Compounds.
From www.pinterest.com
This graphic looks at several properties of elements in the Periodic Boiling Point Trends Of Organic Compounds The size of a molecule influences its. Boiling points increase as the number of carbons is increased. The intermolecular forces increase with increasing polarization (i.e. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to. Boiling Point Trends Of Organic Compounds.
From www.engineeringtoolbox.com
Boiling points of hydrocarbons, alcohols and acids Boiling Point Trends Of Organic Compounds The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. Predict the relative boil points of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. An understanding of the various types of noncovalent forces allows us to explain, on a molecular. Boiling Point Trends Of Organic Compounds.
From labbyag.es
Melting And Boiling Points Of Compounds Chart Labb by AG Boiling Point Trends Of Organic Compounds The size of a molecule influences its. The stronger the imfs, the lower the vapor pressure of the. Boiling points are a measure of intermolecular forces. The intermolecular forces increase with increasing polarization (i.e. Predict the relative boil points of organic compounds. Boiling points increase as the number of carbons is increased. An understanding of the various types of noncovalent. Boiling Point Trends Of Organic Compounds.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds The stronger the imfs, the lower the vapor pressure of the. Intermolecular forces (imfs) can be used to predict relative boiling points. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points are a measure of intermolecular forces. Difference in electronegativity) of bonds. The intermolecular forces increase with increasing polarization. Boiling Point Trends Of Organic Compounds.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds The size of a molecule influences its. Boiling points are a measure of intermolecular forces. The stronger the imfs, the lower the vapor pressure of the. Intermolecular forces (imfs) can be used to predict relative boiling points. The intermolecular forces increase with increasing polarization (i.e. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows. Boiling Point Trends Of Organic Compounds.
From estudarpara.com
Predict the trend in boiling points for the following compounds (1 Boiling Point Trends Of Organic Compounds Predict the relative boil points of organic compounds. Boiling points increase as the number of carbons is increased. Difference in electronegativity) of bonds. The size of a molecule influences its. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. An understanding of the various types of noncovalent forces allows us to. Boiling Point Trends Of Organic Compounds.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation, free download ID1562148 Boiling Point Trends Of Organic Compounds Boiling points are a measure of intermolecular forces. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. Physical properties of organic compounds. Predict the relative boil points of organic compounds. Boiling points increase. Boiling Point Trends Of Organic Compounds.
From wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Boiling Point Trends Of Organic Compounds Boiling points are a measure of intermolecular forces. Intermolecular forces (imfs) can be used to predict relative boiling points. Boiling points increase as the number of carbons is increased. The size of a molecule influences its. Predict the relative boil points of organic compounds. Physical properties of organic compounds. An understanding of the various types of noncovalent forces allows us. Boiling Point Trends Of Organic Compounds.
From cabinet.matttroy.net
Melting And Boiling Point Periodic Table Trends Matttroy Boiling Point Trends Of Organic Compounds Boiling points increase as the number of carbons is increased. The size of a molecule influences its. Intermolecular forces (imfs) can be used to predict relative boiling points. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Physical properties of organic compounds. An understanding of the various types of noncovalent forces. Boiling Point Trends Of Organic Compounds.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Boiling Point Trends Of Organic Compounds Boiling points are a measure of intermolecular forces. The stronger the imfs, the lower the vapor pressure of the. The size of a molecule influences its. Difference in electronegativity) of bonds. Predict the relative boil points of organic compounds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Intermolecular forces (imfs). Boiling Point Trends Of Organic Compounds.
From www.coursehero.com
[Solved] Explain the following trend in the boiling points of the Boiling Point Trends Of Organic Compounds The stronger the imfs, the lower the vapor pressure of the. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Predict the relative boil points of organic compounds. The size of a molecule influences its. Boiling points increase as the number of carbons is increased. Boiling points are a measure of. Boiling Point Trends Of Organic Compounds.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Boiling Point Trends Of Organic Compounds An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Intermolecular forces (imfs) can be used to predict relative boiling points. The intermolecular forces increase with increasing polarization (i.e. The size of a molecule influences its. The stronger the imfs, the lower the vapor pressure of the. Physical properties of organic compounds.. Boiling Point Trends Of Organic Compounds.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID2942238 Boiling Point Trends Of Organic Compounds The size of a molecule influences its. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Intermolecular forces (imfs) can be used to predict relative boiling points. Boiling points are a measure of intermolecular forces. Difference in electronegativity) of bonds. Physical properties of organic compounds. Predict the relative boil points of. Boiling Point Trends Of Organic Compounds.
From www.youtube.com
Comparing the Boiling Point of Alkanes Organic Chemistry YouTube Boiling Point Trends Of Organic Compounds Boiling points increase as the number of carbons is increased. Predict the relative boil points of organic compounds. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The intermolecular forces increase with increasing polarization (i.e. Intermolecular forces (imfs) can be used to predict relative boiling points.. Boiling Point Trends Of Organic Compounds.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Trends Of Organic Compounds An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. The intermolecular forces increase with increasing polarization (i.e. The stronger the imfs, the lower the vapor pressure of the. Boiling points are a measure of intermolecular forces. An understanding of the various types of noncovalent forces allows us to explain, on a. Boiling Point Trends Of Organic Compounds.
From www.youtube.com
Boiling points of organic compounds Structure and bonding Organic Boiling Point Trends Of Organic Compounds The intermolecular forces increase with increasing polarization (i.e. Predict the relative boil points of organic compounds. The size of a molecule influences its. Physical properties of organic compounds. Difference in electronegativity) of bonds. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many. Boiling points are a measure of intermolecular forces. Intermolecular. Boiling Point Trends Of Organic Compounds.