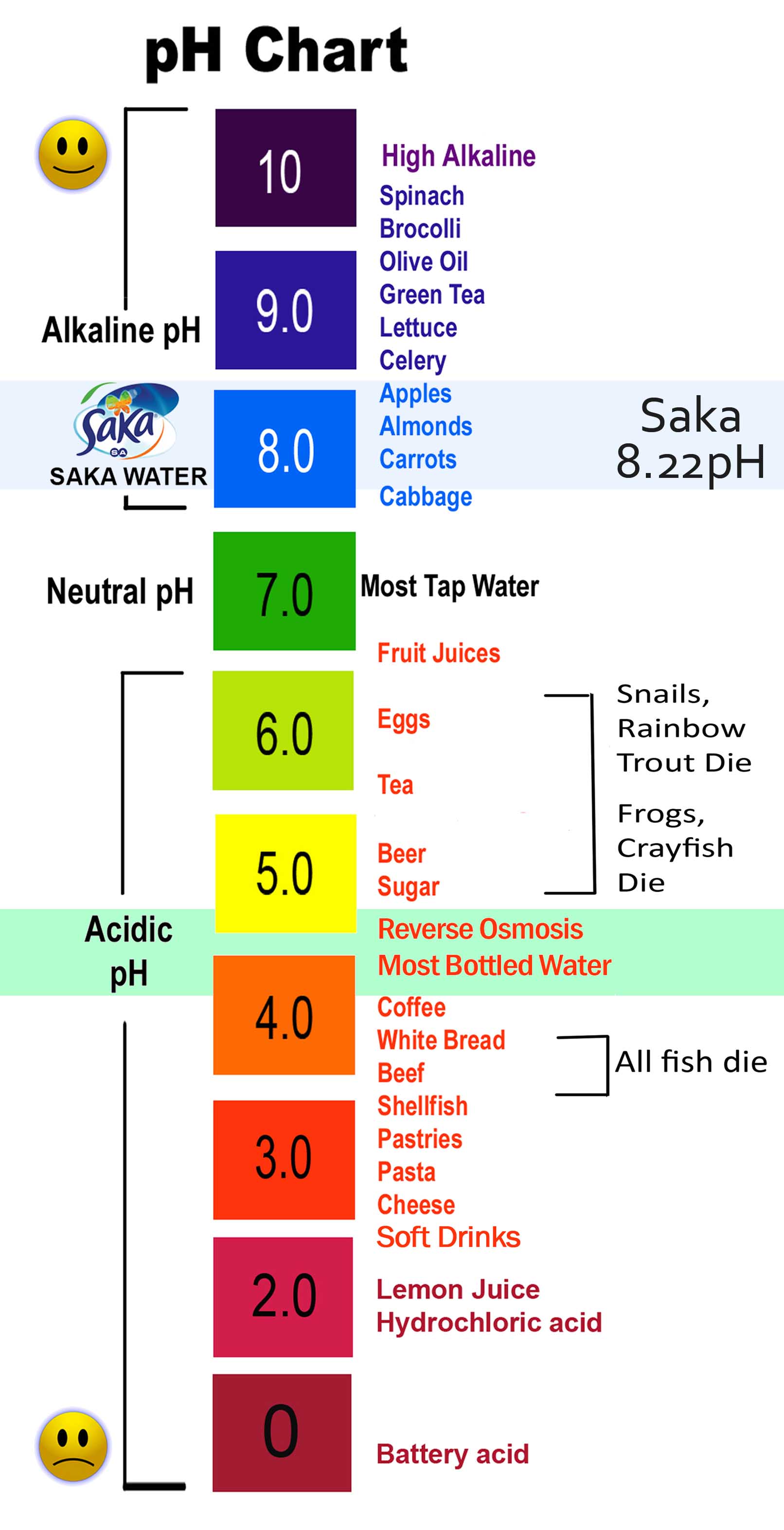
Soaps Are An Example Acid Or Base . No, soap is not an acid. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. Soil ph ranges anywhere from 3 to 10. On a ph scale, substances that form compounds with potassium or sodium. The ph of a detergent. It is a salt that is formed by the reaction between an acid and a base. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. This is because the process. In conclusion, soap is neither an acid nor a base. Detergents are compounds of sodium or potassium hydroxides. Soaps are formed by the combination of strong bases and weak acids i.e. The ph of a soap depends on other ingredients in its formula. Neutral compounds are denoted by 7. So, don’t get too caught up in the.
from amchemistryblog.wordpress.com
Neutral compounds are denoted by 7. Soil ph ranges anywhere from 3 to 10. Soaps are formed by the combination of strong bases and weak acids i.e. It is a salt that is formed by the reaction between an acid and a base. The ph of a detergent. In conclusion, soap is neither an acid nor a base. On a ph scale, substances that form compounds with potassium or sodium. The ph of a soap depends on other ingredients in its formula. Detergents are compounds of sodium or potassium hydroxides. No, soap is not an acid.
4a. Acids and Bases Antonia's chemistry blog
Soaps Are An Example Acid Or Base Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. In conclusion, soap is neither an acid nor a base. On a ph scale, substances that form compounds with potassium or sodium. This is because the process. The ph of a soap depends on other ingredients in its formula. It is a salt that is formed by the reaction between an acid and a base. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. The ph of a detergent. Soaps are formed by the combination of strong bases and weak acids i.e. No, soap is not an acid. Soil ph ranges anywhere from 3 to 10. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. Detergents are compounds of sodium or potassium hydroxides. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. The salts formed by the reaction between weak acids. Neutral compounds are denoted by 7.
From wordwall.net
Tart Household Acids and Bases Group sort Soaps Are An Example Acid Or Base Soil ph ranges anywhere from 3 to 10. No, soap is not an acid. Neutral compounds are denoted by 7. It is a salt that is formed by the reaction between an acid and a base. This is because the process. The salts formed by the reaction between weak acids. In conclusion, soap is neither an acid nor a base.. Soaps Are An Example Acid Or Base.
From sciencenotes.org
The pH Scale of Common Chemicals Soaps Are An Example Acid Or Base The ph of a detergent. On a ph scale, substances that form compounds with potassium or sodium. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. This is because the process. Detergents are compounds of. Soaps Are An Example Acid Or Base.
From www.teachoo.com
10+ Acids used in Daily Life (with images) Teachoo Science Soaps Are An Example Acid Or Base Soaps are formed by the combination of strong bases and weak acids i.e. Detergents are compounds of sodium or potassium hydroxides. On a ph scale, substances that form compounds with potassium or sodium. Soil ph ranges anywhere from 3 to 10. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what. Soaps Are An Example Acid Or Base.
From cosmowholesalepk.com
SOAP BASES Archives Cosmo Wholesale Soaps Are An Example Acid Or Base So, don’t get too caught up in the. The ph of a soap depends on other ingredients in its formula. Soil ph ranges anywhere from 3 to 10. The ph of a detergent. No, soap is not an acid. This is because the process. Neutral compounds are denoted by 7. On a ph scale, substances that form compounds with potassium. Soaps Are An Example Acid Or Base.
From cartoondealer.com
Ph Scale Acids And Alkalines Examples Stock Photo Soaps Are An Example Acid Or Base Detergents are compounds of sodium or potassium hydroxides. The ph of a detergent. Soaps are formed by the combination of strong bases and weak acids i.e. Soil ph ranges anywhere from 3 to 10. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. On a ph scale, acid to base runs. Soaps Are An Example Acid Or Base.
From giotimlbq.blob.core.windows.net
Identifying Acids And Bases Lab at Jeffery Garcia blog Soaps Are An Example Acid Or Base Detergents are compounds of sodium or potassium hydroxides. Neutral compounds are denoted by 7. Soaps are formed by the combination of strong bases and weak acids i.e. This is because the process. The salts formed by the reaction between weak acids. So, don’t get too caught up in the. On a ph scale, substances that form compounds with potassium or. Soaps Are An Example Acid Or Base.
From gioqrcqxb.blob.core.windows.net
Vinegar Borax Dish Soap Weed Killer Recipe at Amy Ohler blog Soaps Are An Example Acid Or Base This is because the process. So, don’t get too caught up in the. It is a salt that is formed by the reaction between an acid and a base. No, soap is not an acid. The salts formed by the reaction between weak acids. The ph of a soap depends on other ingredients in its formula. Soaps are formed by. Soaps Are An Example Acid Or Base.
From www.lisabronner.com
Skin Health, pH, and Dr. Bronner's Soap Going Green Soaps Are An Example Acid Or Base On a ph scale, substances that form compounds with potassium or sodium. This is because the process. Soil ph ranges anywhere from 3 to 10. The ph of a detergent. The ph of a soap depends on other ingredients in its formula. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6. Soaps Are An Example Acid Or Base.
From gioneiyrl.blob.core.windows.net
Acids And Bases Lab Questions at Jenifer Baker blog Soaps Are An Example Acid Or Base No, soap is not an acid. It is a salt that is formed by the reaction between an acid and a base. The ph of a detergent. The ph of a soap depends on other ingredients in its formula. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known. Soaps Are An Example Acid Or Base.
From acidsandbasesrios.weebly.com
pH Acids and Bases Soaps Are An Example Acid Or Base It is a salt that is formed by the reaction between an acid and a base. So, don’t get too caught up in the. Detergents are compounds of sodium or potassium hydroxides. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to. Soaps Are An Example Acid Or Base.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub Soaps Are An Example Acid Or Base Neutral compounds are denoted by 7. This is because the process. It is a salt that is formed by the reaction between an acid and a base. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. Detergents are compounds of sodium or potassium hydroxides. The salts formed by the reaction between. Soaps Are An Example Acid Or Base.
From laundrydetergentideas.com
Is Soap Basic or Acidic in Property Soaps Are An Example Acid Or Base So, don’t get too caught up in the. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. It is a salt that is formed by the reaction between an acid and a base. No, soap. Soaps Are An Example Acid Or Base.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base Soaps Are An Example Acid Or Base No, soap is not an acid. Detergents are compounds of sodium or potassium hydroxides. The ph of a detergent. Soaps are formed by the combination of strong bases and weak acids i.e. It is a salt that is formed by the reaction between an acid and a base. In conclusion, soap is neither an acid nor a base. So, don’t. Soaps Are An Example Acid Or Base.
From guitarscalechart.z28.web.core.windows.net
acids and bases ph scale chart 3.12 acids and bases human biology Soaps Are An Example Acid Or Base On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. No, soap is not an acid. The ph of a detergent. The salts formed by the reaction between weak acids. On a ph scale, substances that form. Soaps Are An Example Acid Or Base.
From www.secure.facebook.com
Kojic Acid Soaps for sale in Burgersfort, Mpumalanga, South Africa Soaps Are An Example Acid Or Base Detergents are compounds of sodium or potassium hydroxides. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. The ph of a soap depends on other ingredients in its formula. So, don’t get too caught up in the. Neutral compounds are denoted by 7. Soil ph ranges anywhere from 3 to 10.. Soaps Are An Example Acid Or Base.
From cbseadda.blogspot.mx
CBSE Chemistry 7th Acids bases and Salts [Solved] CBSE ADDA Soaps Are An Example Acid Or Base On a ph scale, substances that form compounds with potassium or sodium. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. This is because the process. The ph of a detergent. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked. Soaps Are An Example Acid Or Base.
From studyfinder.org
Understanding Acids and Bases Exploring Chemistry Worksheet Answers Soaps Are An Example Acid Or Base The ph of a soap depends on other ingredients in its formula. Soaps are formed by the combination of strong bases and weak acids i.e. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. Soap is. Soaps Are An Example Acid Or Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Soaps Are An Example Acid Or Base Soil ph ranges anywhere from 3 to 10. So, don’t get too caught up in the. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. Detergents are compounds of sodium or potassium hydroxides. The salts formed by the reaction between weak acids. Soaps are formed by the combination of strong bases. Soaps Are An Example Acid Or Base.
From giotimlbq.blob.core.windows.net
Identifying Acids And Bases Lab at Jeffery Garcia blog Soaps Are An Example Acid Or Base It is a salt that is formed by the reaction between an acid and a base. The salts formed by the reaction between weak acids. So, don’t get too caught up in the. Detergents are compounds of sodium or potassium hydroxides. This is because the process. Soil ph ranges anywhere from 3 to 10. No, soap is not an acid.. Soaps Are An Example Acid Or Base.
From gioaztzst.blob.core.windows.net
Is Dove Soap A Toilet Soap at Hannah Farr blog Soaps Are An Example Acid Or Base This is because the process. It is a salt that is formed by the reaction between an acid and a base. The ph of a soap depends on other ingredients in its formula. On a ph scale, substances that form compounds with potassium or sodium. The ph of a detergent. Neutral compounds are denoted by 7. The salts formed by. Soaps Are An Example Acid Or Base.
From techiescientist.com
Is Soap Acidic or Basic? Techiescientist Soaps Are An Example Acid Or Base Soaps are formed by the combination of strong bases and weak acids i.e. On a ph scale, substances that form compounds with potassium or sodium. The ph of a soap depends on other ingredients in its formula. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. It is a salt that. Soaps Are An Example Acid Or Base.
From giotimlbq.blob.core.windows.net
Identifying Acids And Bases Lab at Jeffery Garcia blog Soaps Are An Example Acid Or Base Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. Soap is a salt of a fatty acid, and it is typically slightly basic (alkaline) rather than acidic. In conclusion, soap is neither an acid nor. Soaps Are An Example Acid Or Base.
From giooshzkp.blob.core.windows.net
Soap Bar Smells at Glenn Waggoner blog Soaps Are An Example Acid Or Base So, don’t get too caught up in the. Soil ph ranges anywhere from 3 to 10. No, soap is not an acid. The ph of a detergent. The ph of a soap depends on other ingredients in its formula. On a ph scale, substances that form compounds with potassium or sodium. Soaps are formed by the combination of strong bases. Soaps Are An Example Acid Or Base.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog Soaps Are An Example Acid Or Base On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. So, don’t get too caught up in the. The ph of a detergent. Detergents are compounds of sodium or potassium hydroxides. Soaps are formed by the combination. Soaps Are An Example Acid Or Base.
From crosswordlabs.com
CLASS 7 COMMON ACIDS AND BASES Crossword Labs Soaps Are An Example Acid Or Base The ph of a soap depends on other ingredients in its formula. Soaps are formed by the combination of strong bases and weak acids i.e. The ph of a detergent. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic. Soaps Are An Example Acid Or Base.
From sciencenotes.org
Household Acids and Bases Soaps Are An Example Acid Or Base On a ph scale, substances that form compounds with potassium or sodium. In conclusion, soap is neither an acid nor a base. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. No, soap is not an. Soaps Are An Example Acid Or Base.
From www.gauthmath.com
Solved Key Ideas Acids and Bases Acids, Bases, and the pH Scale The Soaps Are An Example Acid Or Base In conclusion, soap is neither an acid nor a base. The salts formed by the reaction between weak acids. No, soap is not an acid. This is because the process. Soaps are formed by the combination of strong bases and weak acids i.e. It is a salt that is formed by the reaction between an acid and a base. On. Soaps Are An Example Acid Or Base.
From studyfinder.org
Understanding Acids and Bases Exploring Chemistry Worksheet Answers Soaps Are An Example Acid Or Base Soil ph ranges anywhere from 3 to 10. It is a salt that is formed by the reaction between an acid and a base. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as bases. On a ph. Soaps Are An Example Acid Or Base.
From kidspressmagazine.com
Acids and Bases Soaps Are An Example Acid Or Base Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. Soil ph ranges anywhere from 3 to 10. Neutral compounds are denoted by 7. In conclusion, soap is neither an acid nor a base. No, soap. Soaps Are An Example Acid Or Base.
From gioneiyrl.blob.core.windows.net
Acids And Bases Lab Questions at Jenifer Baker blog Soaps Are An Example Acid Or Base The ph of a soap depends on other ingredients in its formula. The ph of a detergent. The salts formed by the reaction between weak acids. Soil ph ranges anywhere from 3 to 10. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a. Soaps Are An Example Acid Or Base.
From homeclasp.com
Is Liquid Soap an Acid or Base? Soaps Are An Example Acid Or Base So, don’t get too caught up in the. Detergents are compounds of sodium or potassium hydroxides. The ph of a soap depends on other ingredients in its formula. In conclusion, soap is neither an acid nor a base. The ph of a detergent. On a ph scale, substances that form compounds with potassium or sodium. Soil ph ranges anywhere from. Soaps Are An Example Acid Or Base.
From crosswordlabs.com
Acids & Bases Crossword Labs Soaps Are An Example Acid Or Base It is a salt that is formed by the reaction between an acid and a base. This is because the process. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the ph scale. Soil ph ranges anywhere from. Soaps Are An Example Acid Or Base.
From crosswordlabs.com
acid bases and salts Crossword Labs Soaps Are An Example Acid Or Base The ph of a detergent. In conclusion, soap is neither an acid nor a base. It is a salt that is formed by the reaction between an acid and a base. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is. Soaps Are An Example Acid Or Base.
From gioneiyrl.blob.core.windows.net
Acids And Bases Lab Questions at Jenifer Baker blog Soaps Are An Example Acid Or Base This is because the process. Soaps are formed by the combination of strong bases and weak acids i.e. In conclusion, soap is neither an acid nor a base. On a ph scale, acid to base runs 1 to 14 wherein compounds ranging from 1 to 6 are marked as acids while those ranging between 8 to 14 are termed as. Soaps Are An Example Acid Or Base.
From www.secure.facebook.com
Kojic Acid Soaps for sale in Burgersfort, Mpumalanga, South Africa Soaps Are An Example Acid Or Base Soil ph ranges anywhere from 3 to 10. Neutral compounds are denoted by 7. On a ph scale, substances that form compounds with potassium or sodium. In conclusion, soap is neither an acid nor a base. This is because the process. No, soap is not an acid. It is a salt that is formed by the reaction between an acid. Soaps Are An Example Acid Or Base.