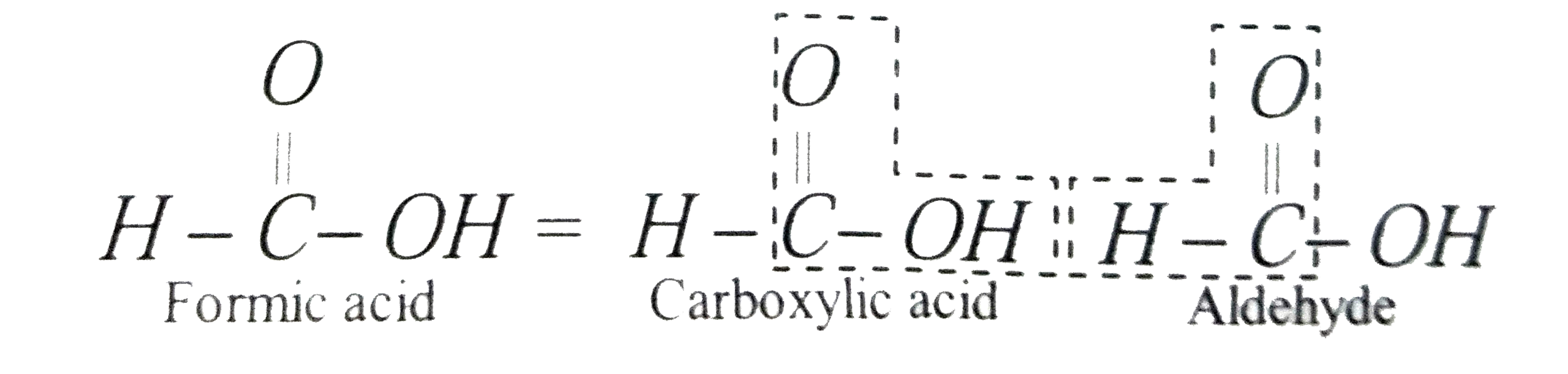Reagent To Test For Carboxylic Acid . A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. test for carboxylic acids. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid.
from www.doubtnut.com
Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. test for carboxylic acids. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and.
The carboxylic acid which reduces Tollen's reagent is
Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. test for carboxylic acids. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid.
From www.organicchemistrytutor.com
Reactions of Carboxylic Acids — Organic Chemistry Tutor Reagent To Test For Carboxylic Acid the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. Carboxylic acids will react with. Reagent To Test For Carboxylic Acid.
From www.researchgate.net
Scheme 2 Synthesis of carboxylic acid 5[12]. Reagents and conditions Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. test for carboxylic acids. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. when carboxylic. Reagent To Test For Carboxylic Acid.
From www.doubtnut.com
Which reagent directly converts carboxylic acids to alcohols? Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon. Reagent To Test For Carboxylic Acid.
From www.transformationtutoring.com
Synthesis And Reactions Of Carboxylic Acids Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. test for. Reagent To Test For Carboxylic Acid.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Reagent To Test For Carboxylic Acid Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. tollens’. Reagent To Test For Carboxylic Acid.
From www.etsy.com
Carboxylic Acids Reagents and Mechanism Guide Organic Etsy Reagent To Test For Carboxylic Acid when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. A) reaction of carboxylic acid with water to produce a slightly acidic. Reagent To Test For Carboxylic Acid.
From www.youtube.com
Synthesis of Carboxylic Acids Carboxylation of Grignard Reagents YouTube Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. test for carboxylic acids. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. identify aldehydes, ketones, alcohols, carboxylic acids,. Reagent To Test For Carboxylic Acid.
From www.youtube.com
Preparation of Carboxylic Acids from Grignard Reagents, Chemistry Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. A) reaction of carboxylic acid with water. Reagent To Test For Carboxylic Acid.
From www.nagwa.com
Question Video Recognizing Carboxylic Acid Esterification from the Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. Carboxylic acids will react with metal. Reagent To Test For Carboxylic Acid.
From www.youtube.com
Test for Carboxylic Acids YouTube Reagent To Test For Carboxylic Acid the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide.. Reagent To Test For Carboxylic Acid.
From www.toppr.com
Explain the preparation of carboxylic acids from Grignard reagent. Give Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences. Reagent To Test For Carboxylic Acid.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Reagent To Test For Carboxylic Acid Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph. Reagent To Test For Carboxylic Acid.
From www.chemistrysteps.com
Aldehydes and Ketones to CArboxylic Acids Chemistry Steps Reagent To Test For Carboxylic Acid the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the hydrazone is usually a brightly colored yellow, orange or red compound, so. Reagent To Test For Carboxylic Acid.
From www.numerade.com
Classification Tests for Carboxylic Acids and Their Derivatives Write Reagent To Test For Carboxylic Acid the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. when carboxylic acid reacts. Reagent To Test For Carboxylic Acid.
From www.doubtnut.com
The carboxylic acid which reduces Tollen's reagent is Reagent To Test For Carboxylic Acid when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. A) reaction of carboxylic acid with water to. Reagent To Test For Carboxylic Acid.
From www.chemistrystudent.com
Carboxylic Acids (ALevel) ChemistryStudent Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. Carboxylic acids will react with metal carbonates. Reagent To Test For Carboxylic Acid.
From www.youtube.com
Preparation of Carboxylic Acids from Grignard reagent, Alkene & Alkyl Reagent To Test For Carboxylic Acid when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. test for carboxylic. Reagent To Test For Carboxylic Acid.
From onlinelibrary.wiley.com
Carboxylic Acid Salts as Dual‐Function Reagents for Carboxylation and Reagent To Test For Carboxylic Acid test for carboxylic acids. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. Carboxylic acids will. Reagent To Test For Carboxylic Acid.
From chem.libretexts.org
Oxidation by Chromic Acid Chemistry LibreTexts Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. test for carboxylic acids. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and.. Reagent To Test For Carboxylic Acid.
From mungfali.com
Grignard Reagent With Carboxylic Acid Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. test for carboxylic acids. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. the hydrazone is usually a brightly colored yellow, orange or red. Reagent To Test For Carboxylic Acid.
From www.chegg.com
Classification Tests for Carboxylic Acids and Their Reagent To Test For Carboxylic Acid when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. test for carboxylic acids. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. tollens’ reagent. Reagent To Test For Carboxylic Acid.
From www.chemistryviews.org
Safer Reagent for the Direct Amidation of Carboxylic Acids ChemistryViews Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. tollens’ reagent oxidizes an aldehyde into the. Reagent To Test For Carboxylic Acid.
From www.doubtnut.com
Carboxylic acids are synthesised from Grignard reagent by the action o Reagent To Test For Carboxylic Acid Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid.. Reagent To Test For Carboxylic Acid.
From www.masterorganicchemistry.com
Reducing Sugars — Master Organic Chemistry Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. test for carboxylic acids. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. identify. Reagent To Test For Carboxylic Acid.
From www.youtube.com
Functional Group Identification Test for Carboxylic Acid YouTube Reagent To Test For Carboxylic Acid the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. tollens’ reagent oxidizes an aldehyde into the. Reagent To Test For Carboxylic Acid.
From www.youtube.com
test of carboxylic acid YouTube Reagent To Test For Carboxylic Acid A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c) results of a positive ph test. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. test for carboxylic acids. when carboxylic. Reagent To Test For Carboxylic Acid.
From www.chemistrysteps.com
Carboxylic Acids and Their Derivatives Practice Problems Chemistry Steps Reagent To Test For Carboxylic Acid Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. test for carboxylic acids. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. . Reagent To Test For Carboxylic Acid.
From chemistry-europe.onlinelibrary.wiley.com
Direct Addition of Grignard Reagents to Aliphatic Carboxylic Acids Reagent To Test For Carboxylic Acid test for carboxylic acids. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. A) reaction. Reagent To Test For Carboxylic Acid.
From app.jove.com
Preparation of Carboxylic Acids Carboxylation of Grignard Reagents Reagent To Test For Carboxylic Acid when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. test for carboxylic acids. tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. the functional groups that will be analyzed in this experiment. Reagent To Test For Carboxylic Acid.
From www.studocu.com
Test for Carboxylic Acid TEST FOR CARBOXYLIC ACIDS EXPERIMENT 10 DATA Reagent To Test For Carboxylic Acid the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. test for carboxylic acids. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. identify aldehydes,. Reagent To Test For Carboxylic Acid.
From www.numerade.com
SOLVED Devise the most efficient synthesis for the carboxylic acid Reagent To Test For Carboxylic Acid identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is. Reagent To Test For Carboxylic Acid.
From askfilo.com
The carboxylic acid which reduces Tollen's reagent is Filo Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. the hydrazone is usually a brightly colored yellow, orange or red compound, so the reaction is often used to test for the presences of. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. when carboxylic acid reacts with sodium bicarbonate solution. Reagent To Test For Carboxylic Acid.
From byjus.com
Grignard reagent does nt react with carboxylic acid Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c). Reagent To Test For Carboxylic Acid.
From www.studypool.com
SOLUTION Carboxylic acids test Studypool Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. A) reaction of carboxylic acid with water to produce a slightly acidic solution, b) results of a negative ph test, c). Reagent To Test For Carboxylic Acid.
From www.researchgate.net
Coupling of carboxylic acids with alcohols using PhIO as a coupling Reagent To Test For Carboxylic Acid tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic acid. identify aldehydes, ketones, alcohols, carboxylic acids, alkenes and. the functional groups that will be analyzed in this experiment are alcohols, phenols, aldehydes, ketones, carboxylic acids, and. when carboxylic acid reacts with sodium bicarbonate solution carbon dioxide is evolved with a brisk effervescence along. A) reaction of. Reagent To Test For Carboxylic Acid.