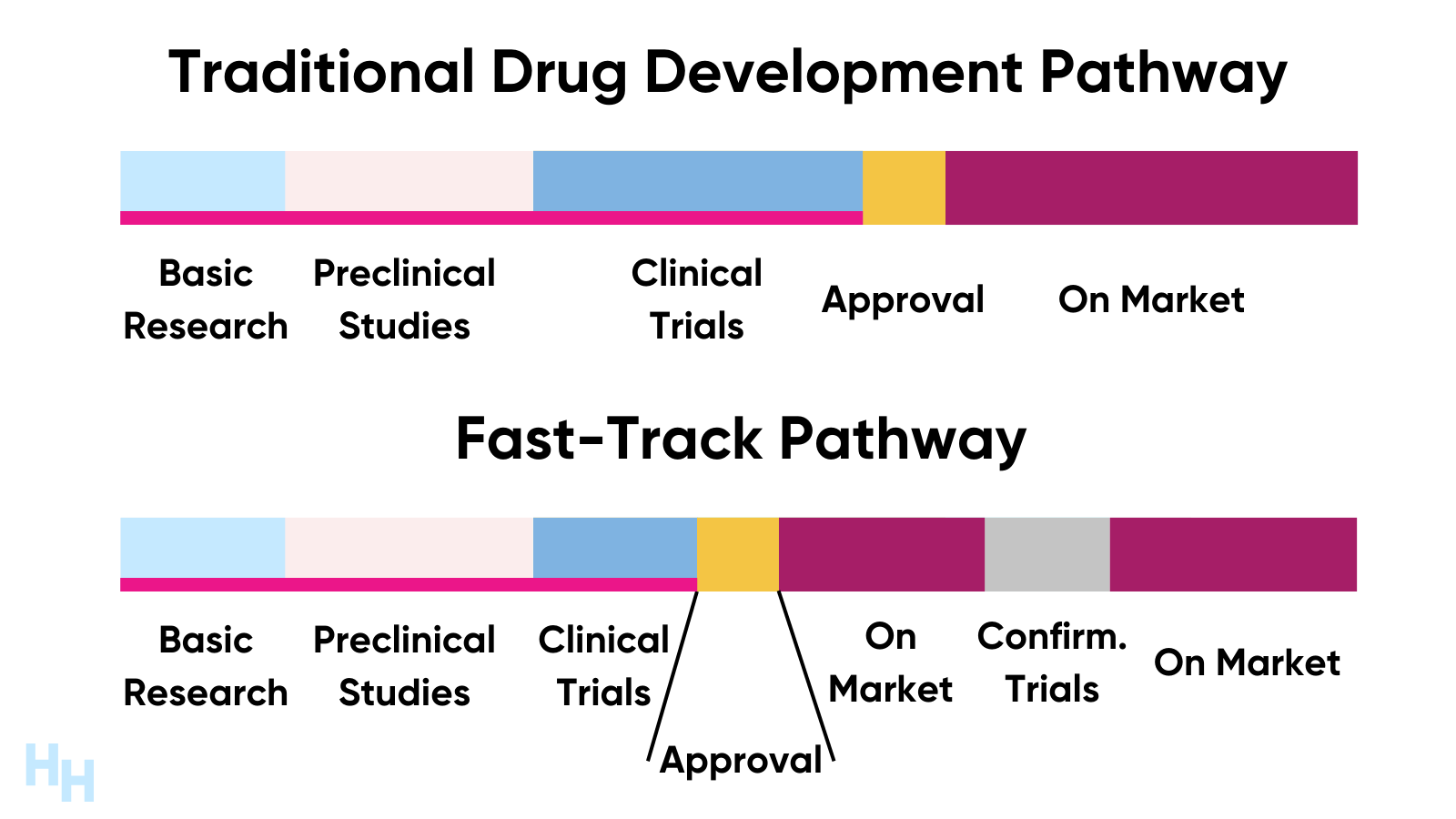
What Is Fast Track Designation By Fda . fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. The added focus, along with. A process designed to expedite the development and review of drugs which may demonstrate. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base.
from workweek.com
fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with.
FastTrack Approval Balancing Speed and Safety in Drug Approvals
What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A process designed to expedite the development and review of drugs which may demonstrate.
From lupusnewstoday.com
RC18 Receives FDA's Fast Track Designation for SLE Treatment What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. fast track is a. What Is Fast Track Designation By Fda.
From lifesciences.csoftintl.com
FDA Grants Fast Track Designation to Syndax Pharmaceuticals What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. fast track is a process designed to facilitate the development and expedite the review of new drugs and.. What Is Fast Track Designation By Fda.
From en.antaranews.com
Biosyngen Announces FDA Fast Track Designation for BST02 in Treatment What Is Fast Track Designation By Fda A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. the fast. What Is Fast Track Designation By Fda.
From www.slideshare.net
FDAFast Track Designation What Is Fast Track Designation By Fda A process designed to expedite the development and review of drugs which may demonstrate. The added focus, along with. fast track is a process designed to facilitate the development and expedite the review of new drugs and. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track. What Is Fast Track Designation By Fda.
From www.bibamedtech.com
FDA grants FastTrack designation to develop heart failure drug BIBA What Is Fast Track Designation By Fda section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track is a process designed to facilitate the development and expedite the review of new drugs and. . What Is Fast Track Designation By Fda.
From workweek.com
FastTrack Approval Balancing Speed and Safety in Drug Approvals What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. The added focus, along with.. What Is Fast Track Designation By Fda.
From cureibm.org
Arimoclomol Receives FDA Fast Track Designation for Inclusion Body What Is Fast Track Designation By Fda section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track designation allows the. What Is Fast Track Designation By Fda.
From investorshub.advfn.com
Rich Pharmaceuticals Inc. (fka RCHA) RCHA Expects FDA Orphan Drug What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a process designed to. What Is Fast Track Designation By Fda.
From www.scendea.com
Fast Track Designation and Breakthrough Therapy Designation — Scendea What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track. What Is Fast Track Designation By Fda.
From medicircle.in
Fast Track designation facilitates development of new therapies that What Is Fast Track Designation By Fda section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. fast track is a process designed to facilitate the development and expedite the review of new drugs and. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A. What Is Fast Track Designation By Fda.
From www.pulmonologyadvisor.com
Inhaled Molgramostim Granted Fast Track Status for Autoimmune Pulmonary What Is Fast Track Designation By Fda The added focus, along with. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. the fast track designation sends a positive signal not only to patients, physicians, and insurers but. What Is Fast Track Designation By Fda.
From checkrare.com
FDA Grants Fast Track Designation for Neurofibromatosis Type 1 Therapy What Is Fast Track Designation By Fda A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. the fast track designation sends a positive signal not. What Is Fast Track Designation By Fda.
From www.modality-solutions.com
FDA’s Fast Track Approval Coronavirus Treatment Acceleration Program What Is Fast Track Designation By Fda section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review. What Is Fast Track Designation By Fda.
From www.dotmed.com
FDA grants Fast Track designation to 64CuDotatate What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. . What Is Fast Track Designation By Fda.
From thelansis.com
FDA Grants Fast Track Designation to MorphoSys AG's Promising What Is Fast Track Designation By Fda A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. fast track is a process designed to facilitate the. What Is Fast Track Designation By Fda.
From thelansis.com
FDA Grants Fast Track Designation to Bayer's Asundexian for Stroke What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat.. What Is Fast Track Designation By Fda.
From arci.org
Vutrisiran receives Fast Track Designation from FDA Amyloidosis What Is Fast Track Designation By Fda fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. A process designed to expedite the development and review of drugs which may demonstrate. The added focus, along with. the fast. What Is Fast Track Designation By Fda.
From www.reddit.com
MESO granted FDA FastTrack Designation for ARDS r/ATHX What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. The added focus, along with. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track. What Is Fast Track Designation By Fda.
From www.linkedin.com
Petra Rietschel MD PhD on LinkedIn BioNTech Receives FDA Fast Track What Is Fast Track Designation By Fda fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. The added focus, along with. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. the fast track designation. What Is Fast Track Designation By Fda.
From fdareporter.com
BIOMARIN Pioneer in Phenylketonuria (PKU) and Gene Therapy, Receives What Is Fast Track Designation By Fda The added focus, along with. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a. What Is Fast Track Designation By Fda.
From www.ozmosi.com
Value of FDA Accelerated Approval in Drug Development Ozmosi What Is Fast Track Designation By Fda The added focus, along with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track is a process designed to facilitate the development, and expedite the review. What Is Fast Track Designation By Fda.
From nckpharma.com
What are the major differences between Breakthrough Therapy What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. A process designed to expedite the development and review of drugs which may demonstrate. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. fast track designation allows the. What Is Fast Track Designation By Fda.
From www.appliedclinicaltrialsonline.com
FDA’s Expedited Review Process The Need for Speed What Is Fast Track Designation By Fda section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. A process designed to expedite the development and review of drugs which may demonstrate. . What Is Fast Track Designation By Fda.
From www.slideshare.net
FDAFast Track Designation What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development and expedite the review of new drugs and. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. The added focus, along with. fast track is a process designed to facilitate the development, and expedite the review. What Is Fast Track Designation By Fda.
From bioinformant.com
U.S. FDA Grants Fast Track Designation to Pluristem's PLXPAD for CLI What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A process designed to expedite the development and review of drugs which may demonstrate. fast track designation allows the manufacturer or. What Is Fast Track Designation By Fda.
From lifesciences.csoftintl.com
FDA Grants Fast Track Designation for Gemini Therapeutics’ Treatment GEM103 What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track is a process designed to facilitate the development and expedite the review of new drugs and. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. A. What Is Fast Track Designation By Fda.
From www.scendea.com
Fast Track Designation and Breakthrough Therapy Designation — Scendea What Is Fast Track Designation By Fda fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. The added focus, along with. A process designed to expedite the development and review of drugs which may demonstrate. fast track. What Is Fast Track Designation By Fda.
From en.prnasia.com
Biosyngen Announces FDA Fast Track Designation for BST02 in Treatment What Is Fast Track Designation By Fda A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development and expedite the review of new drugs and. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. the fast track designation sends. What Is Fast Track Designation By Fda.
From checkrare.com
FDA Fast Track Designation for Gene Therapy in CLN1 Disease (Infantile What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. the fast track designation. What Is Fast Track Designation By Fda.
From bioinformant.com
U.S. FDA Grants Fast Track Designation to Pluristem's PLXPAD for CLI What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. section 901 of fdasia amends the federal. What Is Fast Track Designation By Fda.
From www.globenewswire.com
Disc Medicine Receives FDA Fast Track Designation for What Is Fast Track Designation By Fda fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. A process designed to expedite the development and review of drugs which may demonstrate. section 901 of fdasia amends the federal food, drug,. What Is Fast Track Designation By Fda.
From www.painnewsnetwork.org
FDA Gives Fast Track Designation to New Pain Med — Pain News Network What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. The. What Is Fast Track Designation By Fda.
From thelansis.com
FDA Grants Fast Track Designation for Reneo Pharma's Mavodelpar (REN001 What Is Fast Track Designation By Fda the fast track designation sends a positive signal not only to patients, physicians, and insurers but also to. fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. A process designed to expedite the development and review of drugs which may demonstrate. fast track is a process designed to. What Is Fast Track Designation By Fda.
From www.slideshare.net
FDAFast Track Designation What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. section 901 of fdasia amends the federal food, drug, and cosmetic act (fd&c act) to allow the fda to base. The added focus,. What Is Fast Track Designation By Fda.
From www.youtube.com
US FDA Fast Track Designation YouTube What Is Fast Track Designation By Fda fast track is a process designed to facilitate the development, and expedite the review of drugs to treat. fast track is a process designed to facilitate the development and expedite the review of new drugs and. fast track designation allows the manufacturer or drug developer to meet and communicate more frequently with. The added focus, along with.. What Is Fast Track Designation By Fda.