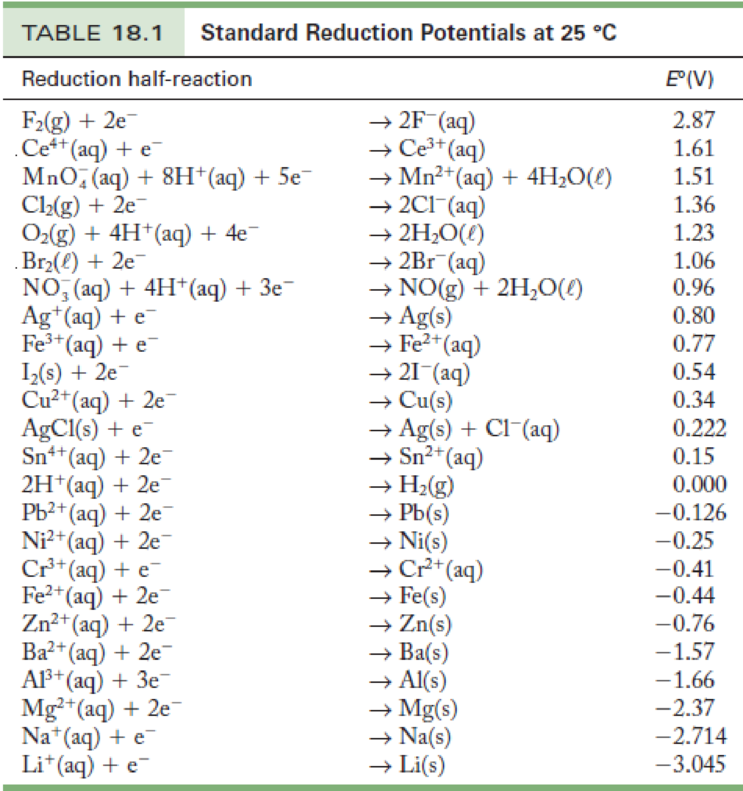
Standard Electrode Potential Of X Y Z Are 0.75 . The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly.
from dinorahiu-images.blogspot.com
The standard cell potential is a measure of the driving force. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following.
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint
Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Of X Y Z Are 0.75 Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential is a measure of the driving force. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Electrode Potential Of X Y Z Are 0.75.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the driving force. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From fyoxffenv.blob.core.windows.net
Standard Electrode Potential Positive Or Negative at David Means blog Standard Electrode Potential Of X Y Z Are 0.75 Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Electrode Potential Of X Y Z Are 0.75 In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution:. Standard Electrode Potential Of X Y Z Are 0.75.
From www.docsity.com
Standard Electrode Potentials General Chemistry CHEM 152 Docsity Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.toppr.com
Standard electrode potential data are useful for understanding the Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Of X Y Z Are 0.75 In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential is a measure of the driving force. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution:. Standard Electrode Potential Of X Y Z Are 0.75.
From scienceinfo.com
Measuring the Standard Electrode Potential (Eꝋ) Standard Electrode Potential Of X Y Z Are 0.75 In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell. Standard Electrode Potential Of X Y Z Are 0.75.
From www.academia.edu
(PDF) Standard Electrode Potential Aubriecia Lim Academia.edu Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution:. Standard Electrode Potential Of X Y Z Are 0.75.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic. Standard Electrode Potential Of X Y Z Are 0.75.
From www.scribd.com
Standard Electrode Potentials Standard Electrode Potential Of X Y Z Are 0.75 Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic. Standard Electrode Potential Of X Y Z Are 0.75.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of. Standard Electrode Potential Of X Y Z Are 0.75.
From www.studocu.com
Standard Electrode Potentials Electrochemistry Studocu Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Electrode Potential Of X Y Z Are 0.75.
From www.numerade.com
Standard electrode potentials example 1 Numerade Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution:. Standard Electrode Potential Of X Y Z Are 0.75.
From studylib.net
19 Applications of Standard Electrode Potentials Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the. Standard Electrode Potential Of X Y Z Are 0.75.
From www.chegg.com
Solved Standard electrode potential, E (in volts) 0.04 Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly.. Standard Electrode Potential Of X Y Z Are 0.75.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Electrode Potential Of X Y Z Are 0.75.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Electrode Potential Of X Y Z Are 0.75.
From users.highland.edu
Standard Potentials Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for. Standard Electrode Potential Of X Y Z Are 0.75.
From questions-in.kunduz.com
51. . The standard electrode potentials f... Physical Chemistry Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following.. Standard Electrode Potential Of X Y Z Are 0.75.
From askfilo.com
The standard electrode potentials, E∘ for the half cell reactions are as Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.studypool.com
SOLUTION Standard electrode potential,definition,explanation and Standard Electrode Potential Of X Y Z Are 0.75 To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Electrode Potential Of X Y Z Are 0.75.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential is a measure of the. Standard Electrode Potential Of X Y Z Are 0.75.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential is a measure of the driving force. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.flinnsci.ca
Standard Reduction Potential Chart Flinn Scientific Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the driving force. To find the difference of the two half cells, the following. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. To find the difference of the two half cells, the following. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of. Standard Electrode Potential Of X Y Z Are 0.75.
From www.youtube.com
Standard electrode potential \( \left(E^{\circ}\right) \) for Standard Electrode Potential Of X Y Z Are 0.75 In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Consider the following table of standard electrode potentials for. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5368514 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. To find the difference of the two half cells, the following. The standard cell potential is a measure of the. Standard Electrode Potential Of X Y Z Are 0.75.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly.. Standard Electrode Potential Of X Y Z Are 0.75.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential is a measure of the driving force. Consider the following table of standard electrode potentials for a series of hypothetical reactions in aqueous solution: To find the difference of the two half cells, the following. In physics,. Standard Electrode Potential Of X Y Z Are 0.75.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential Of X Y Z Are 0.75 The standard cell potential is a measure of the driving force. In physics, the electrochemical potential is often not defined explicitly, instead the electrostatic energy z j fϕ α is included directly. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells,. Standard Electrode Potential Of X Y Z Are 0.75.