What Happens When A Base Is Added To A Solution . Mixing equal amounts of a. A ph equal to 7 indicates a neutral solution, and water is. We get an alkaline solution. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Learn about acids, alkalis, indicators, ph and neutralisation. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Neutralisation is the reaction between an acid and a base to form a salt plus water. We get an acidic solution when an acid is dissolved in water; All solutions in water are either acidic, alkaline or neutral: Learn how acids and bases react to form salts and water, and how to name the salts. They are also known to have a bitter taste as well as a slippery or soapy.
from jackwestin.com
Learn how acids and bases react to form salts and water, and how to name the salts. They are also known to have a bitter taste as well as a slippery or soapy. All solutions in water are either acidic, alkaline or neutral: Learn about acids, alkalis, indicators, ph and neutralisation. A ph equal to 7 indicates a neutral solution, and water is. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Neutralisation is the reaction between an acid and a base to form a salt plus water. Mixing equal amounts of a. We get an acidic solution when an acid is dissolved in water; If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water.
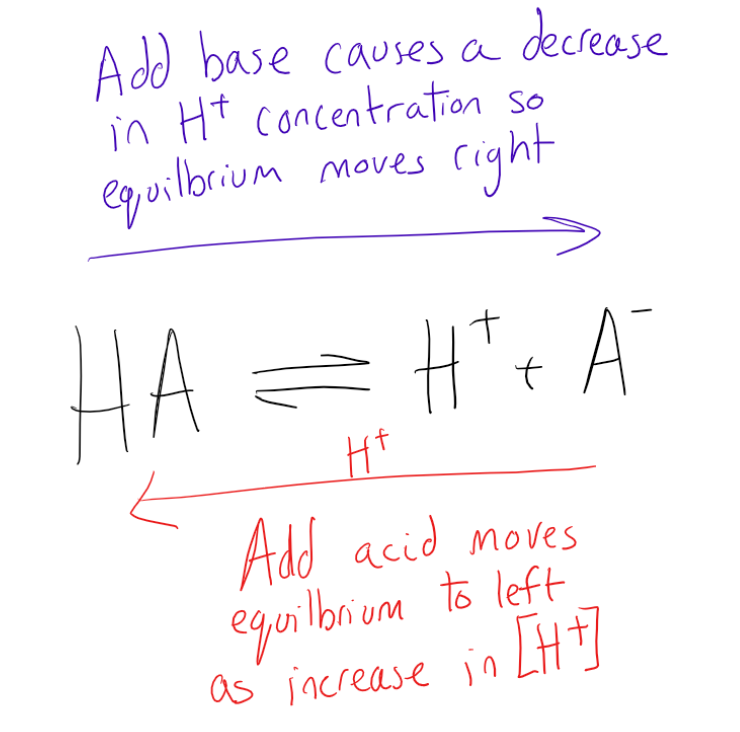
Buffers 2 Acid Base Equilibria MCAT Content
What Happens When A Base Is Added To A Solution They are also known to have a bitter taste as well as a slippery or soapy. Learn how acids and bases react to form salts and water, and how to name the salts. All solutions in water are either acidic, alkaline or neutral: If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Mixing equal amounts of a. Neutralisation is the reaction between an acid and a base to form a salt plus water. We get an alkaline solution. Learn about acids, alkalis, indicators, ph and neutralisation. A ph equal to 7 indicates a neutral solution, and water is. We get an acidic solution when an acid is dissolved in water; They are also known to have a bitter taste as well as a slippery or soapy. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and.
From chem.libretexts.org
4.E Reactions in Aqueous Solution (Exercises) Chemistry LibreTexts What Happens When A Base Is Added To A Solution Learn about acids, alkalis, indicators, ph and neutralisation. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. All solutions in water are either acidic, alkaline or neutral: An acid in a water solution tastes sour, changes the colour of blue litmus paper. What Happens When A Base Is Added To A Solution.
From www.chegg.com
Solved When an acid or a base is added to a buffer, What Happens When A Base Is Added To A Solution We get an acidic solution when an acid is dissolved in water; An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Neutralisation is the reaction between an acid and a base to form a salt plus. What Happens When A Base Is Added To A Solution.
From www.youtube.com
what happens to an acid or base in a water solution? class 10th What Happens When A Base Is Added To A Solution If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Learn about acids, alkalis, indicators, ph and neutralisation. Neutralisation is the reaction between an acid and a base to form a salt plus water. A ph equal to 7 indicates a neutral solution,. What Happens When A Base Is Added To A Solution.
From www.youtube.com
What happens to an acid or a base in water solutionAcids bases and What Happens When A Base Is Added To A Solution If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. We get an alkaline solution. All solutions in water are either acidic, alkaline or neutral: Learn about acids, alkalis, indicators, ph and neutralisation. Learn how acids and bases react to form salts and. What Happens When A Base Is Added To A Solution.
From general.chemistrysteps.com
Acidity of a Salt Solution Chemistry Steps What Happens When A Base Is Added To A Solution Mixing equal amounts of a. A ph equal to 7 indicates a neutral solution, and water is. We get an alkaline solution. Learn how acids and bases react to form salts and water, and how to name the salts. Learn about acids, alkalis, indicators, ph and neutralisation. They are also known to have a bitter taste as well as a. What Happens When A Base Is Added To A Solution.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians What Happens When A Base Is Added To A Solution If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. We get an acidic solution when an acid is dissolved in water; Learn about acids, alkalis, indicators, ph and neutralisation. A ph equal to 7 indicates a neutral solution, and water is. They. What Happens When A Base Is Added To A Solution.
From www.youtube.com
What Happens to an Acid or a Base in a Water Solution? Activity 2.9 What Happens When A Base Is Added To A Solution We get an acidic solution when an acid is dissolved in water; Mixing equal amounts of a. All solutions in water are either acidic, alkaline or neutral: If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. We get an alkaline solution. Learn. What Happens When A Base Is Added To A Solution.
From www.alchem.ie
Equilibrium & Acid Base by Sarah Wegwerth What Happens When A Base Is Added To A Solution An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. They are also known to have a bitter taste as well as a slippery or soapy. All solutions in water are either acidic, alkaline or neutral: We. What Happens When A Base Is Added To A Solution.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Happens When A Base Is Added To A Solution An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. We get an alkaline solution. Learn about acids, alkalis, indicators, ph and neutralisation. Mixing equal amounts of a. If you mix equal amounts of a strong acid. What Happens When A Base Is Added To A Solution.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Happens When A Base Is Added To A Solution Learn about acids, alkalis, indicators, ph and neutralisation. All solutions in water are either acidic, alkaline or neutral: An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. We get an alkaline solution. They are also known. What Happens When A Base Is Added To A Solution.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and What Happens When A Base Is Added To A Solution All solutions in water are either acidic, alkaline or neutral: An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Neutralisation is the reaction between an acid and a base to form a salt plus water. We. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Is Added To A Solution If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. A ph equal to 7 indicates a neutral solution, and water is. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals. What Happens When A Base Is Added To A Solution.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples What Happens When A Base Is Added To A Solution We get an acidic solution when an acid is dissolved in water; A ph equal to 7 indicates a neutral solution, and water is. Neutralisation is the reaction between an acid and a base to form a salt plus water. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo What Happens When A Base Is Added To A Solution All solutions in water are either acidic, alkaline or neutral: Learn how acids and bases react to form salts and water, and how to name the salts. Mixing equal amounts of a. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. A. What Happens When A Base Is Added To A Solution.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Happens When A Base Is Added To A Solution A ph equal to 7 indicates a neutral solution, and water is. All solutions in water are either acidic, alkaline or neutral: We get an acidic solution when an acid is dissolved in water; An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen,. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
MCQ What happens when a solution of an acid is mixed with a solution What Happens When A Base Is Added To A Solution A ph equal to 7 indicates a neutral solution, and water is. All solutions in water are either acidic, alkaline or neutral: If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. We get an alkaline solution. Learn about acids, alkalis, indicators, ph. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Happens When A Base Is Added To A Solution An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Neutralisation is the reaction between an acid and a base to form a salt plus water. Mixing equal amounts of a. All solutions in water are either. What Happens When A Base Is Added To A Solution.
From www.youtube.com
What Happens to an Acid or a Base in Water Solution Class 10 What Happens When A Base Is Added To A Solution A ph equal to 7 indicates a neutral solution, and water is. Learn how acids and bases react to form salts and water, and how to name the salts. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. They are also known. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
How does a Base react in Water (aqueous solution)? Teachoo Chemistry What Happens When A Base Is Added To A Solution They are also known to have a bitter taste as well as a slippery or soapy. A ph equal to 7 indicates a neutral solution, and water is. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts,. What Happens When A Base Is Added To A Solution.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content What Happens When A Base Is Added To A Solution We get an alkaline solution. Neutralisation is the reaction between an acid and a base to form a salt plus water. We get an acidic solution when an acid is dissolved in water; If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water.. What Happens When A Base Is Added To A Solution.
From www.gauthmath.com
Solved According to the Arrhenius model of acids and bases, what What Happens When A Base Is Added To A Solution We get an acidic solution when an acid is dissolved in water; They are also known to have a bitter taste as well as a slippery or soapy. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts,. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Is Added To A Solution An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Learn about acids, alkalis, indicators, ph and neutralisation. All solutions in water are either acidic, alkaline or neutral: Learn how acids and bases react to form salts. What Happens When A Base Is Added To A Solution.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry What Happens When A Base Is Added To A Solution Learn about acids, alkalis, indicators, ph and neutralisation. A ph equal to 7 indicates a neutral solution, and water is. We get an acidic solution when an acid is dissolved in water; We get an alkaline solution. Learn how acids and bases react to form salts and water, and how to name the salts. Neutralisation is the reaction between an. What Happens When A Base Is Added To A Solution.
From lessonlibrarysnotter.z13.web.core.windows.net
Identify The Neutralization Reaction What Happens When A Base Is Added To A Solution A ph equal to 7 indicates a neutral solution, and water is. Neutralisation is the reaction between an acid and a base to form a salt plus water. Learn how acids and bases react to form salts and water, and how to name the salts. All solutions in water are either acidic, alkaline or neutral: Mixing equal amounts of a.. What Happens When A Base Is Added To A Solution.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry What Happens When A Base Is Added To A Solution Learn how acids and bases react to form salts and water, and how to name the salts. They are also known to have a bitter taste as well as a slippery or soapy. A ph equal to 7 indicates a neutral solution, and water is. We get an acidic solution when an acid is dissolved in water; We get an. What Happens When A Base Is Added To A Solution.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Happens When A Base Is Added To A Solution We get an alkaline solution. Learn about acids, alkalis, indicators, ph and neutralisation. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Learn how acids and bases react to form salts and water, and how to. What Happens When A Base Is Added To A Solution.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Happens When A Base Is Added To A Solution Learn how acids and bases react to form salts and water, and how to name the salts. A ph equal to 7 indicates a neutral solution, and water is. They are also known to have a bitter taste as well as a slippery or soapy. Neutralisation is the reaction between an acid and a base to form a salt plus. What Happens When A Base Is Added To A Solution.
From saylordotorg.github.io
Reactions in Aqueous Solution What Happens When A Base Is Added To A Solution All solutions in water are either acidic, alkaline or neutral: A ph equal to 7 indicates a neutral solution, and water is. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Neutralisation is the reaction between an acid and a base to. What Happens When A Base Is Added To A Solution.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Happens When A Base Is Added To A Solution Learn about acids, alkalis, indicators, ph and neutralisation. Mixing equal amounts of a. All solutions in water are either acidic, alkaline or neutral: An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Neutralisation is the reaction. What Happens When A Base Is Added To A Solution.
From www.youtube.com
Chemistry Acid or base in water solution Acids, bases and salts What Happens When A Base Is Added To A Solution We get an alkaline solution. Learn about acids, alkalis, indicators, ph and neutralisation. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. All solutions in water are either acidic, alkaline or neutral: A ph equal to. What Happens When A Base Is Added To A Solution.
From saylordotorg.github.io
Acids and Bases What Happens When A Base Is Added To A Solution If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. A ph equal to 7 indicates a neutral solution, and water is. Learn about acids, alkalis, indicators, ph and neutralisation. An acid in a water solution tastes sour, changes the colour of blue. What Happens When A Base Is Added To A Solution.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044 What Happens When A Base Is Added To A Solution Learn how acids and bases react to form salts and water, and how to name the salts. They are also known to have a bitter taste as well as a slippery or soapy. Mixing equal amounts of a. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and. What Happens When A Base Is Added To A Solution.
From www.numerade.com
SOLVED Complete the proton transfer equilibrium reaction for the What Happens When A Base Is Added To A Solution They are also known to have a bitter taste as well as a slippery or soapy. A ph equal to 7 indicates a neutral solution, and water is. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts,. What Happens When A Base Is Added To A Solution.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Happens When A Base Is Added To A Solution They are also known to have a bitter taste as well as a slippery or soapy. We get an acidic solution when an acid is dissolved in water; An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts,. What Happens When A Base Is Added To A Solution.
From courses.lumenlearning.com
Buffers Chemistry Atoms First What Happens When A Base Is Added To A Solution An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and. Mixing equal amounts of a. They are also known to have a bitter taste as well as a slippery or soapy. All solutions in water are either. What Happens When A Base Is Added To A Solution.