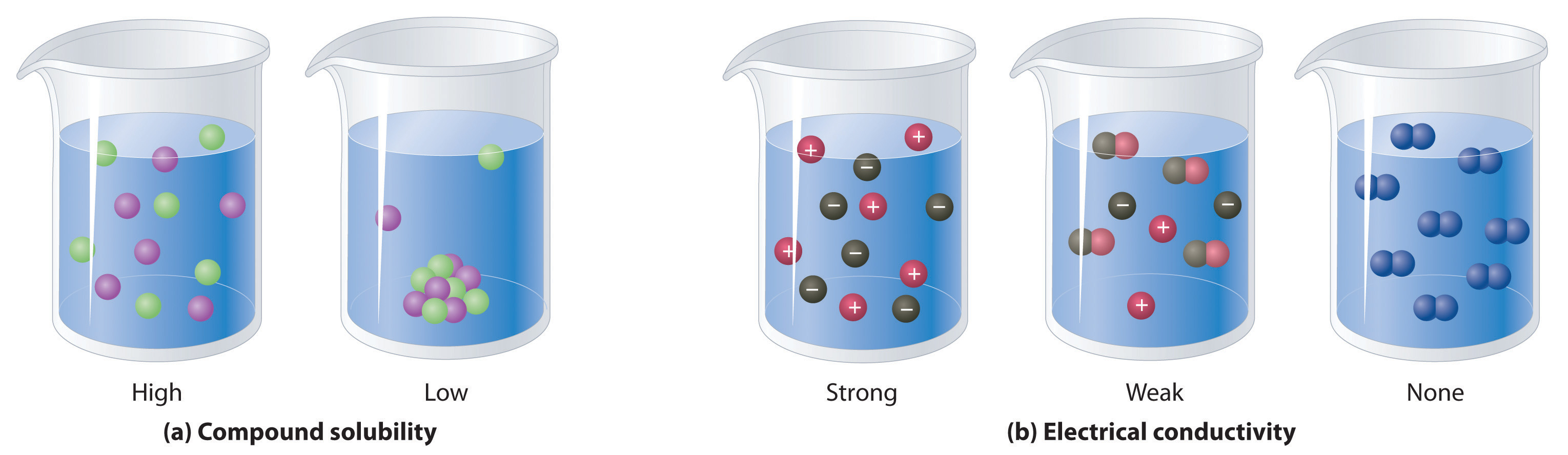
Solid-Liquid Solution Example . Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: In other words, they have infinite mutual solubility. Hydration entropy can make a difference!. Table 11.1 gives examples of several. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. There are seven main types of solutions, this list includes the types and examples: Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Solutions of liquids in liquids. Some liquids may be mixed in any proportions to yield solutions; Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute.
from chem.libretexts.org
Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions of liquids in liquids. In other words, they have infinite mutual solubility. Some liquids may be mixed in any proportions to yield solutions; There are seven main types of solutions, this list includes the types and examples: Table 11.1 gives examples of several. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute.
4.2 Aqueous Solutions Chemistry LibreTexts
Solid-Liquid Solution Example Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions of liquids in liquids. Table 11.1 gives examples of several. Hydration entropy can make a difference!. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. There are seven main types of solutions, this list includes the types and examples: Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. In other words, they have infinite mutual solubility. Some liquids may be mixed in any proportions to yield solutions; Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions.
From courses.lumenlearning.com
Phases and Classification of Matter Chemistry Solid-Liquid Solution Example Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Solutions of liquids in liquids. In other words, they have infinite mutual solubility. Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. There are seven main types of solutions,. Solid-Liquid Solution Example.
From slideplayer.com
Solutions Chapter ppt download Solid-Liquid Solution Example Some liquids may be mixed in any proportions to yield solutions; Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions of liquids in liquids. Table 13.2.1 lists some common examples of gaseous, liquid, and. Solid-Liquid Solution Example.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid-Liquid Solution Example Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Hydration entropy can make a difference!. In other words, they have infinite mutual solubility. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions can be solid, liquid, or gas (liquid solutions are most interesting. Solid-Liquid Solution Example.
From fr.dreamstime.com
Solution Solide Dans Le Liquide Illustration de Vecteur Illustration Solid-Liquid Solution Example Table 11.1 gives examples of several. Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Solutions of liquids in liquids. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in. Solid-Liquid Solution Example.
From www.youtube.com
SOLIDLIQUID SOLUTION AND RAOULT'S LAW YouTube Solid-Liquid Solution Example In other words, they have infinite mutual solubility. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions of liquids in liquids. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to. Solid-Liquid Solution Example.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid-Liquid Solution Example Solutions of liquids in liquids. Hydration entropy can make a difference!. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). There are seven main types of solutions, this list includes the types and examples: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Table 13.2.1 lists. Solid-Liquid Solution Example.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Solid-Liquid Solution Example Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Solutions of liquids in liquids. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Hydration entropy can make a difference!. Some liquids may be mixed in any proportions to yield solutions; Table 13.2.1 lists some common examples. Solid-Liquid Solution Example.
From www.youtube.com
Solubility of solids in liquids YouTube Solid-Liquid Solution Example Hydration entropy can make a difference!. There are seven main types of solutions, this list includes the types and examples: Some liquids may be mixed in any proportions to yield solutions; Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Gas phase solutions are easily formed from any mixture of gases since the. Solid-Liquid Solution Example.
From www.snexplores.org
Explainer What are the different states of matter? Solid-Liquid Solution Example Solutions of liquids in liquids. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. There are seven main types of solutions, this list includes the types and examples: Oxygen (a gas), alcohol (a liquid), and. Solid-Liquid Solution Example.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Solid-Liquid Solution Example Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Some liquids may be mixed in. Solid-Liquid Solution Example.
From sciencenotes.org
Liquid Definition Examples of Liquids Solid-Liquid Solution Example Some liquids may be mixed in any proportions to yield solutions; In other words, they have infinite mutual solubility. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Hydration entropy can make a difference!. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Solutions. Solid-Liquid Solution Example.
From www.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary Solid-Liquid Solution Example There are seven main types of solutions, this list includes the types and examples: In other words, they have infinite mutual solubility. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Solutions can. Solid-Liquid Solution Example.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Solid-Liquid Solution Example Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Some liquids may be mixed in any proportions to yield solutions; Hydration entropy can make a difference!. There are seven main types of solutions, this list includes the types and examples: Solutions can be solid, liquid, or gas (liquid solutions. Solid-Liquid Solution Example.
From slideplayer.com
N39 Properties of Solutions ppt download Solid-Liquid Solution Example Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Some liquids may be mixed in any proportions to yield solutions; Gas phase solutions are easily formed from any mixture. Solid-Liquid Solution Example.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Solid-Liquid Solution Example Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). There are seven main types of solutions, this list includes the types and examples: Table 11.1 gives examples of several. Hydration entropy can make a difference!. In other words, they have infinite mutual solubility. Gas phase solutions are easily formed from any mixture of gases since. Solid-Liquid Solution Example.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid-Liquid Solution Example Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions of liquids in liquids. Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Oxygen. Solid-Liquid Solution Example.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Solid-Liquid Solution Example Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Hydration entropy can make a difference!. In other words, they have infinite mutual solubility. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Two common examples illustrate the contrast between exothermic and endothermic heats of. Solid-Liquid Solution Example.
From www.youtube.com
Solids, Liquids and Gases Class 5 SCIENCE CBSE/NCERT Solutions Solid-Liquid Solution Example Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Some liquids may be mixed in any proportions to yield solutions; In other words, they have infinite mutual solubility. Table 11.1 gives examples of several. Solutions. Solid-Liquid Solution Example.
From www.dreamstime.com
Solution Solid in liquid stock vector. Illustration of mixtures Solid-Liquid Solution Example Hydration entropy can make a difference!. There are seven main types of solutions, this list includes the types and examples: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). In other words, they have infinite mutual solubility.. Solid-Liquid Solution Example.
From chem.libretexts.org
4.2 Aqueous Solutions Chemistry LibreTexts Solid-Liquid Solution Example Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions of liquids in liquids. In other words, they have infinite mutual solubility. Table 11.1 gives examples of several. There are seven main types of solutions,. Solid-Liquid Solution Example.
From www.youtube.com
Factors Affecting Solubility of Solid in Liquid Solution and Solid-Liquid Solution Example There are seven main types of solutions, this list includes the types and examples: In other words, they have infinite mutual solubility. Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Table 11.1 gives examples of several. Two common examples illustrate the contrast between exothermic and endothermic heats of. Solid-Liquid Solution Example.
From sites.google.com
9/8/15 Miss Day's Science Class Blog Solid-Liquid Solution Example Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions of liquids in liquids. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). In other words, they have. Solid-Liquid Solution Example.
From www.youtube.com
solubility of solid in liquid solution chemistry 12th YouTube Solid-Liquid Solution Example Table 11.1 gives examples of several. There are seven main types of solutions, this list includes the types and examples: Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Hydration entropy can make a difference!. In other words, they have infinite mutual solubility. Solutions can be solid, liquid, or. Solid-Liquid Solution Example.
From www.youtube.com
SOLUTION OF SOLID IN LIQUID SOLUTIONll Class Solid-Liquid Solution Example Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Solutions of liquids in liquids. Some liquids may be mixed in any proportions to yield solutions; In other words, they. Solid-Liquid Solution Example.
From examples.yourdictionary.com
Common Examples of Solutions Science in Everyday Life YourDictionary Solid-Liquid Solution Example In other words, they have infinite mutual solubility. There are seven main types of solutions, this list includes the types and examples: Hydration entropy can make a difference!. Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Solutions can be solid, liquid, or gas (liquid solutions are most interesting. Solid-Liquid Solution Example.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid-Liquid Solution Example There are seven main types of solutions, this list includes the types and examples: Hydration entropy can make a difference!. Some liquids may be mixed in any proportions to yield solutions; Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions can be solid, liquid, or gas (liquid solutions are most. Solid-Liquid Solution Example.
From www.youtube.com
9 Types of Solution Chemistry YouTube Solid-Liquid Solution Example Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Hydration entropy can make a difference!. Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Table 11.1 gives examples of several. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so.. Solid-Liquid Solution Example.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid-Liquid Solution Example Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Table 11.1 gives examples of several. Some liquids may be mixed in any proportions to yield solutions; There are seven main types of solutions, this list includes the types and examples: Solutions of liquids in liquids. Solutions can be solid,. Solid-Liquid Solution Example.
From www.youtube.com
Solid in liquid solution YouTube Solid-Liquid Solution Example Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Solutions of liquids in liquids. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Some liquids may be mixed in any proportions to yield solutions; Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies. Solid-Liquid Solution Example.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID6655701 Solid-Liquid Solution Example In other words, they have infinite mutual solubility. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Hydration entropy can make a difference!. Table 13.2.1 lists some common examples of gaseous, liquid, and. Solid-Liquid Solution Example.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube Solid-Liquid Solution Example Table 11.1 gives examples of several. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Gas phase solutions are. Solid-Liquid Solution Example.
From sciencenotes.org
Precipitation Reaction Definition and Examples in Chemistry Solid-Liquid Solution Example Some liquids may be mixed in any proportions to yield solutions; Solutions of liquids in liquids. Hydration entropy can make a difference!. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Table 13.2.1 lists some common examples of gaseous, liquid, and solid solutions and identifies the physical states of the solute. Solutions can. Solid-Liquid Solution Example.
From www.slideserve.com
PPT Chapter 13 Properties of Solutions PowerPoint Presentation, free Solid-Liquid Solution Example Hydration entropy can make a difference!. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: There are seven main types of solutions, this list includes the types and examples: Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Solutions of liquids in liquids. Table. Solid-Liquid Solution Example.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Solid-Liquid Solution Example Solutions of liquids in liquids. Some liquids may be mixed in any proportions to yield solutions; There are seven main types of solutions, this list includes the types and examples: In other words, they have infinite mutual solubility. Gas phase solutions are easily formed from any mixture of gases since the molecules of the gas so. Hydration entropy can make. Solid-Liquid Solution Example.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Solid-Liquid Solution Example Solutions can be solid, liquid, or gas (liquid solutions are most interesting to chemists). Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Some liquids may be mixed in any proportions to yield solutions; Table 11.1 gives examples of several. Solutions of liquids in liquids. Gas phase solutions are. Solid-Liquid Solution Example.