Heat Capacity Of The Calorimeter . The calorimetry calculator can help you solve complex calorimetry problems. For example, when an exothermic. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate heat, temperature change, and specific heat after thermal equilibrium. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Additionally, it can find the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is.
from www.tec-science.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Additionally, it can find the. Calculate heat, temperature change, and specific heat after thermal equilibrium. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. For example, when an exothermic.
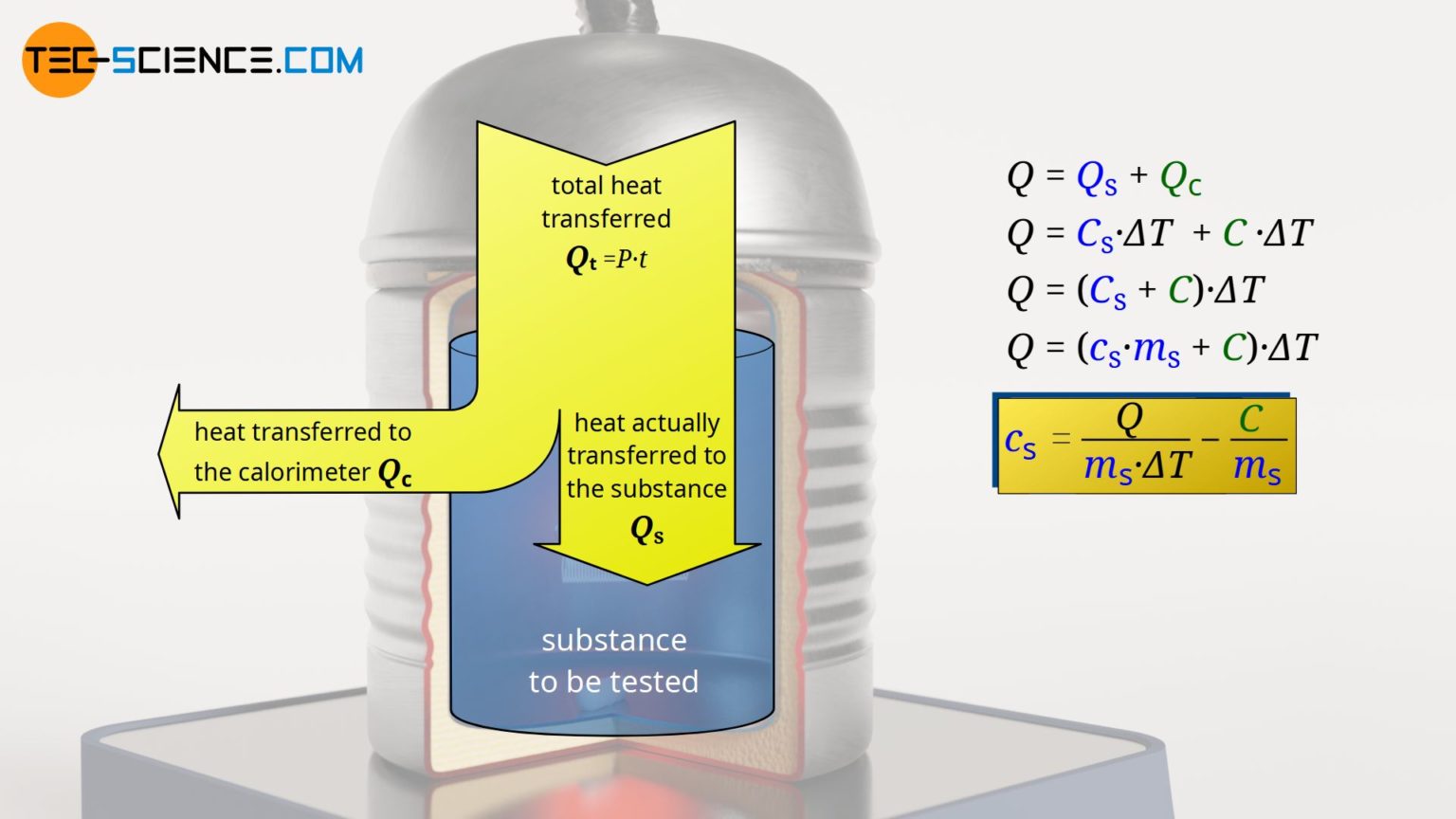
Calorimeter to determine the specific heat capacities of liquids tec
Heat Capacity Of The Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium. The calorimetry calculator can help you solve complex calorimetry problems. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Heat Capacity Of The Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Compare heat flow from hot to. Heat Capacity Of The Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Capacity Of The Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Where q is the amount of heat according to the change in temperature measured in joules. Heat Capacity Of The Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Heat Capacity Of The Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The calorimetry calculator can help you solve complex calorimetry problems. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Heat Capacity Of The Calorimeter.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity Of The Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. A calorimeter is a device used to measure the amount of heat involved in. Heat Capacity Of The Calorimeter.
From www.toppr.com
In a constant volume calorimeter, 3.5 g of a gas with molecular weight Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Calculate heat, temperature change, and specific heat after thermal equilibrium. For example, when an exothermic. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter. Heat Capacity Of The Calorimeter.
From users.highland.edu
Calorimetry Heat Capacity Of The Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A container that prevents heat transfer in or out is called. Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter. Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this Heat Capacity Of The Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. For example, when an exothermic. The calorimetry calculator can help you solve complex calorimetry problems.. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved ACTIVITY 1 A Heat Capacity of the Calorimeter 1. Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter. Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Of The Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Where q is the. Heat Capacity Of The Calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Of The Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat,. Heat Capacity Of The Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Heat Capacity Of The Calorimeter Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Additionally, it can find the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Where q is the amount. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Heat Capacity Of The Calorimeter Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. For example, when an exothermic. Additionally, it can find the. Calculate heat, temperature change, and specific heat after thermal equilibrium. It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat.. Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVED Use the References to access important values if needed for Heat Capacity Of The Calorimeter Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A container that. Heat Capacity Of The Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Heat Capacity Of The Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Compare heat flow from hot to cold objects in an ideal. Heat Capacity Of The Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Heat Capacity Of The Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. The calorimetry calculator can help you solve complex calorimetry problems. The combustion of 1 mole of glucose. Heat Capacity Of The Calorimeter.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Additionally, it can find the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs. Heat Capacity Of The Calorimeter.
From chemistrytalk.org
Calorimetry ChemTalk Heat Capacity Of The Calorimeter If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. For example, when an exothermic. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure. Heat Capacity Of The Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Heat Capacity Of The Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Additionally, it can find the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The calorimetry calculator can help you solve complex calorimetry. Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVED When a solid dissolves in water; heat may be evolved or Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The heat capacity. Heat Capacity Of The Calorimeter.
From www.studocu.com
B. Heat Capacity of Calorimeter B. HEAT CAPACITY OF THE CALORIMETER Heat Capacity Of The Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Where q is the amount of heat according to the. Heat Capacity Of The Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Capacity Of The Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Heat Capacity Of The Calorimeter Additionally, it can find the. For example, when an exothermic. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate heat, temperature change, and specific heat after thermal equilibrium. It can analyze the heat exchange between up to 3. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved ACTIVITY 1 A Heat Capacity of the Calorimeter 1. Heat Capacity Of The Calorimeter Additionally, it can find the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. For. Heat Capacity Of The Calorimeter.
From studylib.net
Heat Capacity of Metals PreLab Heat Capacity Of The Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of. Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Of The Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$. Heat Capacity Of The Calorimeter.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Heat Capacity Of The Calorimeter If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. For example, when. Heat Capacity Of The Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Heat Capacity Of The Calorimeter Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the calorimeter which is. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The. Heat Capacity Of The Calorimeter.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Heat Capacity Of The Calorimeter Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate heat, temperature change, and specific heat after thermal equilibrium. For example, when an exothermic. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity). Heat Capacity Of The Calorimeter.
From www.bartleby.com
Answered Table 1 Calorimeter Heat Capacity… bartleby Heat Capacity Of The Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Additionally, it can find the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Of The Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. For example, when an exothermic. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter. Heat Capacity Of The Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube Heat Capacity Of The Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat, temperature change, and specific heat after thermal equilibrium. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results.. Heat Capacity Of The Calorimeter.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Heat Capacity Of The Calorimeter It can analyze the heat exchange between up to 3 objects. For example, when an exothermic. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The Heat Capacity Of The Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. It can analyze the heat exchange. Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , Heat Capacity Of The Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Where. Heat Capacity Of The Calorimeter.