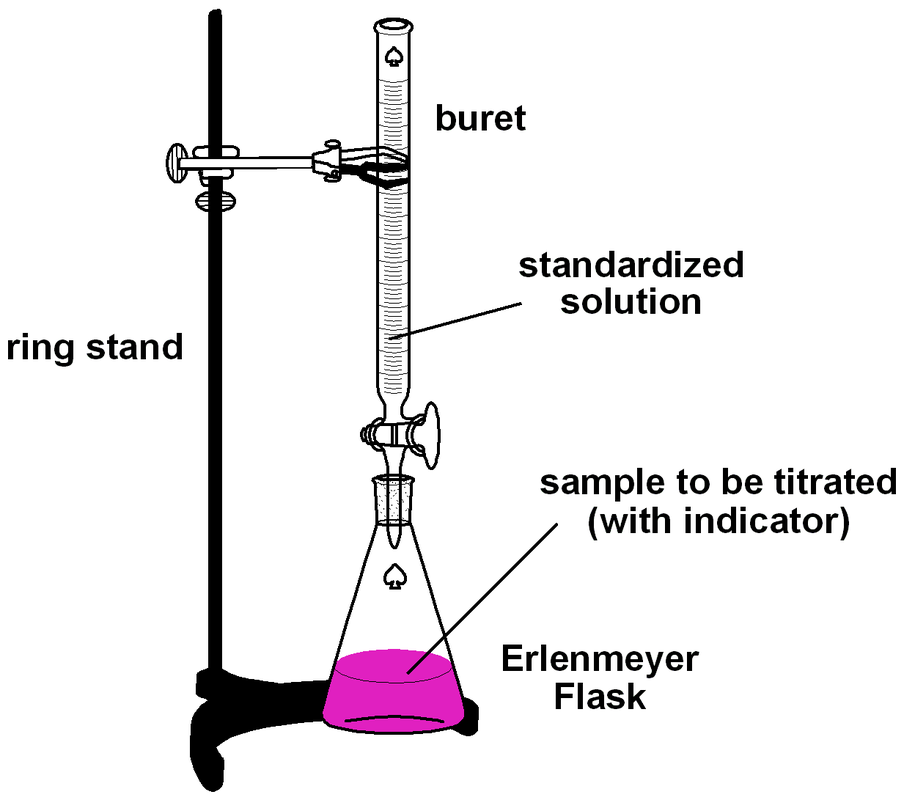
Titration Experiment Vinegar . We will use titration to find the molar concentration. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment, you will perform a series of acid/base titrations. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. You will use 2 ml. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Determine the molarity of acetic. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In the course of these titrations you will become familiar with the technique of titration and the calculations associated.
from pharmaceuticaleducation.blogspot.com
Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). Determine the molarity of acetic. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. We will use titration to find the molar concentration. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. You will use 2 ml. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment, you will perform a series of acid/base titrations.
Pharma gyan Pandit
Titration Experiment Vinegar We will use titration to find the molar concentration. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). Determine the molarity of acetic. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this experiment, you will perform a series of acid/base titrations. We will use titration to find the molar concentration. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. You will use 2 ml.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Titration Experiment Vinegar Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment, you will perform a series of acid/base titrations. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment the percentage of acetic acid present in a white vinegar solution will. Titration Experiment Vinegar.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Experiment Vinegar In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment, you will perform a series of acid/base titrations. You will use 2 ml. We will use titration to find the molar concentration. In this science project, you will determine the amount of acid. Titration Experiment Vinegar.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment the percentage of acetic acid present in a white vinegar. Titration Experiment Vinegar.
From www.youtube.com
CHM 132 / Lab 20 Titration of Vinegar YouTube Titration Experiment Vinegar You will use 2 ml. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). Determine the molarity of acetic. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this experiment we will perform. Titration Experiment Vinegar.
From studylib.net
The Titration of Acetic Acid in Vinegar Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. You will use 2 ml. In the course of these titrations you will become familiar with. Titration Experiment Vinegar.
From www.sciencebuddies.org
Measuring the Amount of Acid in Vinegar by Titration with an Indicator Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by. Titration Experiment Vinegar.
From www.scribd.com
Titration of Vinegar Experiment PDF Titration Chemistry Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In this experiment we will perform a titration, a lab technique that is used to determine. Titration Experiment Vinegar.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration Experiment Vinegar In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. You will use 2 ml. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment, you will perform a series of acid/base titrations. We will use titration to. Titration Experiment Vinegar.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic. Titration Experiment Vinegar.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). We will use titration to find the molar concentration. In this experiment, you will perform a series of acid/base titrations. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined. Titration Experiment Vinegar.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In this experiment we will perform a titration, a lab technique that is used to determine. Titration Experiment Vinegar.
From www.studocu.com
Acetic acid content in vinegar using titration E10 Experiment 10 Titration Experiment Vinegar In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. You will use 2 ml. In the course of these titrations you will become familiar with. Titration Experiment Vinegar.
From www.coursehero.com
[Solved] You have performed a titration of vinegar using a burette and Titration Experiment Vinegar In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution.. Titration Experiment Vinegar.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Experiment Vinegar You will use 2 ml. Determine the molarity of acetic. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Write the balanced equation for. Titration Experiment Vinegar.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this experiment, you will perform a series of acid/base titrations. You will use 2 ml. Determine the molarity of acetic. In this experiment, a technique known as a titration will be used to determine the concentration. Titration Experiment Vinegar.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Titration Experiment Vinegar In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough. Titration Experiment Vinegar.
From www.slideserve.com
PPT Analysis of Vinegar by Titration PowerPoint Presentation, free Titration Experiment Vinegar In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). In the course of these titrations you will become familiar with the technique of titration and. Titration Experiment Vinegar.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Vinegar In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In this experiment, you will perform a series of acid/base titrations. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. Collect a small amount of vinegar in a beaker. Titration Experiment Vinegar.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Experiment Vinegar Determine the molarity of acetic. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. We will use titration to find the molar concentration. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a. Titration Experiment Vinegar.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). We will use titration to find the molar concentration. In this experiment we will. Titration Experiment Vinegar.
From www.studocu.com
Experiment A7 Titration EXPERIMENT A7 VINEGAR TITRATION Learning Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. You will use 2 ml. In this experiment, you will perform a series of acid/base titrations. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute. Titration Experiment Vinegar.
From www.chegg.com
Solved Determination of Acetic Acid Concentration in Vinegar Titration Experiment Vinegar We will use titration to find the molar concentration. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment, you will perform a series of acid/base titrations. Collect. Titration Experiment Vinegar.
From www.sliderbase.com
Titration Titration Experiment Vinegar In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this. Titration Experiment Vinegar.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Experiment Vinegar Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar.. Titration Experiment Vinegar.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Experiment Vinegar In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by. Titration Experiment Vinegar.
From www.scribd.com
Lab Titration of Vinegar Acid Titration Titration Experiment Vinegar In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. In this experiment, you will perform a series of acid/base titrations. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In the course of these. Titration Experiment Vinegar.
From augensternjiang.github.io
Chemistry Experiments and important reactions —— Common experiments Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. We will use titration to find the molar concentration. Determine the molarity of acetic. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. You will. Titration Experiment Vinegar.
From www.vrogue.co
11 Titration Of Vinegar Experiment Chemistry Libretex vrogue.co Titration Experiment Vinegar We will use titration to find the molar concentration. In this experiment, you will perform a series of acid/base titrations. Determine the molarity of acetic. You will use 2 ml. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this experiment the percentage of acetic acid present. Titration Experiment Vinegar.
From www.youtube.com
Titration (using phenolphthalein) YouTube Titration Experiment Vinegar In this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. Determine the molarity of acetic. In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. Write the balanced equation for the neutralization reaction. Titration Experiment Vinegar.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Experiment Vinegar Determine the molarity of acetic. In this experiment, you will perform a series of acid/base titrations. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In this experiment, a technique known as a titration will. Titration Experiment Vinegar.
From www.numerade.com
SOLVED Titlewithtopic Titration Experiment for Acetic Acid in Vinegar Titration Experiment Vinegar We will use titration to find the molar concentration. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Determine the molarity of acetic. In this experiment, you will perform. Titration Experiment Vinegar.
From www.youtube.com
Titration of Vinegar Video Lesson YouTube YouTube Titration Experiment Vinegar We will use titration to find the molar concentration. Determine the molarity of acetic. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In the course of these titrations you will become familiar with the technique of titration and the calculations associated. In this experiment, you will perform. Titration Experiment Vinegar.
From www.vrogue.co
11 Titration Of Vinegar Experiment Chemistry Libretex vrogue.co Titration Experiment Vinegar Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). You will use 2 ml. Determine the molarity of acetic. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. In the course of these titrations you will become familiar with the. Titration Experiment Vinegar.
From www.youtube.com
Vinegar Titration Part A & B General Chemistry Experiment YouTube Titration Experiment Vinegar In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this science project, you will determine the amount of acid in different vinegars using titration, a common technique in chemistry. We will use titration to find the molar concentration. Determine the molarity of acetic. Write the balanced equation. Titration Experiment Vinegar.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Titration Experiment Vinegar In this experiment the percentage of acetic acid present in a white vinegar solution will be determined by titrating it with a known. In this experiment, you will perform a series of acid/base titrations. Collect a small amount of vinegar in a beaker or small flask (each person will need about 15 ml, enough for several titrations). You will use. Titration Experiment Vinegar.