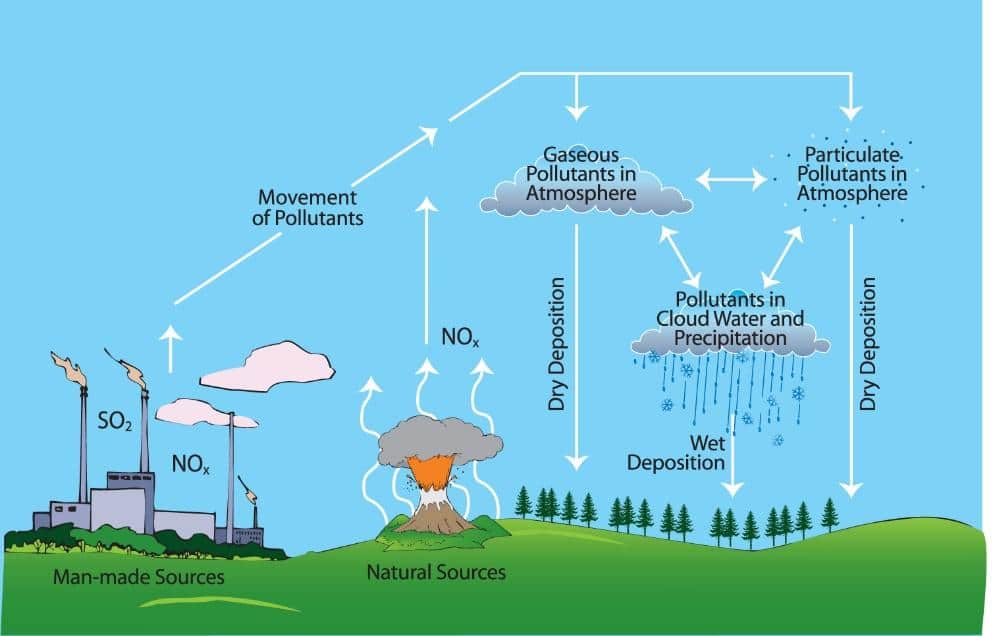
How Does Acid Rain Affect Iron Bridges . The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. Bridges, mining exploration equipment), which are mainly made of steel and. The final step is the reaction of. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity.
from earth.org
The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. Mining equipment and structural elements (e.g. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The most notable effects occur on marble and limestone, which. Bridges, mining exploration equipment), which are mainly made of steel and. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). The final step is the reaction of.
How Does Acid Rain Affect the Environment
How Does Acid Rain Affect Iron Bridges The final step is the reaction of. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. Bridges, mining exploration equipment), which are mainly made of steel and.
From circuitnehajnije39.z21.web.core.windows.net
Chemistry Of Acid Rain Formation How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). As it flows through the soil, acidic rain water can leach aluminum from soil clay. How Does Acid Rain Affect Iron Bridges.
From www.youtube.com
water pollution drawing land pollution drawing acid rain drawing How Does Acid Rain Affect Iron Bridges The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). Mining equipment and structural elements (e.g. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow. How Does Acid Rain Affect Iron Bridges.
From www.greenmatters.com
How Does Acid Rain Affect the Environment? What We Know How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). The zinc oxide converts to zinc hydroxide on exposure to moisture. How Does Acid Rain Affect Iron Bridges.
From ar.inspiredpencil.com
Acid Rain Weathering How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. The most notable effects occur on marble and limestone, which. Mining equipment and structural elements (e.g. Bridges,. How Does Acid Rain Affect Iron Bridges.
From www.careerpower.in
Acid Rain Definition, Effects, and Examples How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. Mining equipment and structural elements (e.g. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. Bridges, mining exploration equipment),. How Does Acid Rain Affect Iron Bridges.
From dokumen.tips
(PPTX) Corrosion of Acid Rain Juliana Lamond Grade 9. The Problem How How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. Bridges, mining exploration equipment), which are mainly made of steel and. The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain,. How Does Acid Rain Affect Iron Bridges.
From www.greenmatters.com
How Does Acid Rain Affect the Environment? What We Know How Does Acid Rain Affect Iron Bridges The final step is the reaction of. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. Bridges, mining exploration equipment), which are mainly made of steel and. Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. As it flows through the soil, acidic rain water. How Does Acid Rain Affect Iron Bridges.
From www.vrogue.co
What Is Acid Rain Acid Precipitation vrogue.co How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The final step is the reaction. How Does Acid Rain Affect Iron Bridges.
From ar.inspiredpencil.com
Acid Rain How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The final step is the reaction of. The most notable effects occur on marble and limestone, which. In order to solve the problem of insufficient accuracy. How Does Acid Rain Affect Iron Bridges.
From earth.org
How Does Acid Rain Affect the Environment How Does Acid Rain Affect Iron Bridges The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of. How Does Acid Rain Affect Iron Bridges.
From testbook.com
[Solved] Acid rain is due to air pollution by How Does Acid Rain Affect Iron Bridges The final step is the reaction of. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Bridges, mining exploration equipment), which are mainly made of steel and. Mining equipment and structural elements (e.g. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In. How Does Acid Rain Affect Iron Bridges.
From letstalkgeography.com
How Does Acid Rain Affect Climate Change? Let's Talk Geography How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem of. How Does Acid Rain Affect Iron Bridges.
From ar.inspiredpencil.com
Acid Precipitation How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). The final step is the reaction of. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The acid rain can destruct. How Does Acid Rain Affect Iron Bridges.
From www.internetgeography.net
What is Acid Rain? Geography How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). As it flows through the soil, acidic rain water can leach aluminum from soil clay. How Does Acid Rain Affect Iron Bridges.
From rain-acid.weebly.com
Causes Acid Rain How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Bridges, mining exploration equipment), which are mainly. How Does Acid Rain Affect Iron Bridges.
From www.vrogue.co
How Does Acid Rain Affect Buildings Statues Pdf Corro vrogue.co How Does Acid Rain Affect Iron Bridges As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Mining equipment and structural elements (e.g. The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem of insufficient accuracy. How Does Acid Rain Affect Iron Bridges.
From www.vrogue.co
Know The Basic Behind Acid Rain And Harmful Effects vrogue.co How Does Acid Rain Affect Iron Bridges Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem. How Does Acid Rain Affect Iron Bridges.
From www.vecteezy.com
Acid rain cycle in nature vector illustration 21669336 Vector Art at How Does Acid Rain Affect Iron Bridges In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The final step is the reaction of. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). The most notable effects occur on marble and limestone, which. Bridges, mining exploration equipment), which are mainly made of steel and.. How Does Acid Rain Affect Iron Bridges.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then. How Does Acid Rain Affect Iron Bridges.
From hxehqntri.blob.core.windows.net
How Does Acid Rain Affect Atmosphere at Paul Thornhill blog How Does Acid Rain Affect Iron Bridges The final step is the reaction of. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Mining equipment and structural elements (e.g. Bridges, mining exploration equipment), which are mainly made of steel and. Buildings and statues are damaged as a result, particularly those made of limestone. How Does Acid Rain Affect Iron Bridges.
From www.vrogue.co
How Does Acid Rain Affect Buildings Statues Pdf Corro vrogue.co How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. Bridges, mining exploration equipment), which are mainly made of steel and. Mining equipment and structural elements (e.g. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The. How Does Acid Rain Affect Iron Bridges.
From www.dreamstime.com
Acid Rain Diagram Stock Illustrations 23 Acid Rain Diagram Stock How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. Mining equipment and structural elements (e.g. Bridges, mining exploration equipment), which are mainly made of steel and. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The zinc oxide converts to zinc hydroxide on exposure to. How Does Acid Rain Affect Iron Bridges.
From mavink.com
Acid Rain Images For Ppt How Does Acid Rain Affect Iron Bridges Mining equipment and structural elements (e.g. Bridges, mining exploration equipment), which are mainly made of steel and. The final step is the reaction of. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Buildings and statues are damaged as a result, particularly those made of limestone. How Does Acid Rain Affect Iron Bridges.
From www.vrogue.co
How Does Acid Rain Form Socratic vrogue.co How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Bridges, mining exploration equipment), which are mainly made of steel and. As it flows through the soil,. How Does Acid Rain Affect Iron Bridges.
From studylib.net
acid rain How does negatively affect the surrounding ecosystem? How Does Acid Rain Affect Iron Bridges The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Bridges, mining exploration equipment), which are mainly made of steel and. The acid rain can destruct the. How Does Acid Rain Affect Iron Bridges.
From earth.org
What is Acid Rain? How Does Acid Rain Affect Iron Bridges The final step is the reaction of. Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity.. How Does Acid Rain Affect Iron Bridges.
From www.breeze-technologies.de
How air pollution causes acid rain Breeze Technologies How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. The final step is the reaction of. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. Bridges, mining exploration. How Does Acid Rain Affect Iron Bridges.
From www.freepik.com
Free Vector Diagram showing acid rain pathway How Does Acid Rain Affect Iron Bridges The most notable effects occur on marble and limestone, which. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Mining equipment and structural elements (e.g. Bridges,. How Does Acid Rain Affect Iron Bridges.
From hxeswywmu.blob.core.windows.net
How Does Acid Precipitation Affect Humans at Kerri Cole blog How Does Acid Rain Affect Iron Bridges The acid rain can destruct the passive film on the iron surface (vértes et al., 1994) and then enhance the dissolution of iron. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Buildings and statues are damaged as a result, particularly those made of limestone (calcium. How Does Acid Rain Affect Iron Bridges.
From www.javatpoint.com
Acid Rain Definition JavaTpoint How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. The most notable effects occur on marble and limestone, which. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain,. How Does Acid Rain Affect Iron Bridges.
From www.cluetrain.co.jp
pamuk Kretanje Morska luka what does acid rain do to steel bridges and How Does Acid Rain Affect Iron Bridges As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Buildings and statues are damaged as a. How Does Acid Rain Affect Iron Bridges.
From exoakmygt.blob.core.windows.net
How Does Acid Rain Affect People's Health at Wight blog How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. Mining equipment and structural elements (e.g.. How Does Acid Rain Affect Iron Bridges.
From www.torquedetail.com
Acid Rain on Car Paint Can Wreck It If You Don't Do This Now How Does Acid Rain Affect Iron Bridges Bridges, mining exploration equipment), which are mainly made of steel and. The most notable effects occur on marble and limestone, which. Buildings and statues are damaged as a result, particularly those made of limestone (calcium carbonate). In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The acid rain can destruct the passive. How Does Acid Rain Affect Iron Bridges.
From exoakmygt.blob.core.windows.net
How Does Acid Rain Affect People's Health at Wight blog How Does Acid Rain Affect Iron Bridges As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. Bridges, mining exploration equipment), which are mainly made of steel and. Mining equipment and structural elements (e.g. The most notable effects occur on marble and limestone, which. The zinc oxide converts to zinc hydroxide on exposure to. How Does Acid Rain Affect Iron Bridges.
From mavink.com
Acid Rain Pathway How Does Acid Rain Affect Iron Bridges The zinc oxide converts to zinc hydroxide on exposure to moisture in the form of rain, dew, or high humidity. The final step is the reaction of. In order to solve the problem of insufficient accuracy and effectiveness of the current analysis model of. The acid rain can destruct the passive film on the iron surface (vértes et al., 1994). How Does Acid Rain Affect Iron Bridges.